Question: A buffer solution is prepared as described in (a) below. To a sample of this buffer solution is added a solution of a strong base
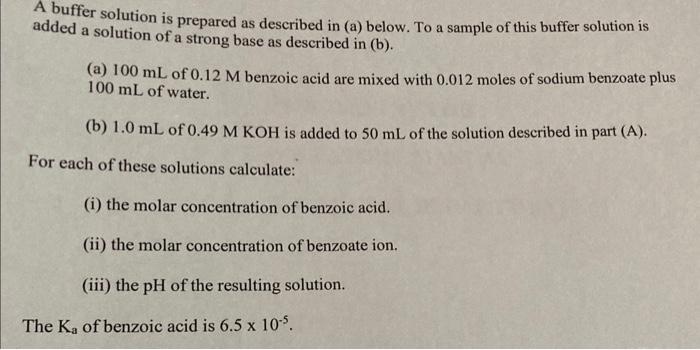
A buffer solution is prepared as described in (a) below. To a sample of this buffer solution is added a solution of a strong base as described in (b). (a) 100mL of 0.12M benzoic acid are mixed with 0.012 moles of sodium benzoate plus 100mL of water. (b) 1.0mL of 0.49MKOH is added to 50mL of the solution described in part (A). For each of these solutions calculate: (i) the molar concentration of benzoic acid. (ii) the molar concentration of benzoate ion. (iii) the pH of the resulting solution. The Ka of benzoic acid is 6.5105
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


