Question: A certain Carnot engine operates between 290 K and 450 K. The engine does 150 J of work for every 600 J of heat absorbed
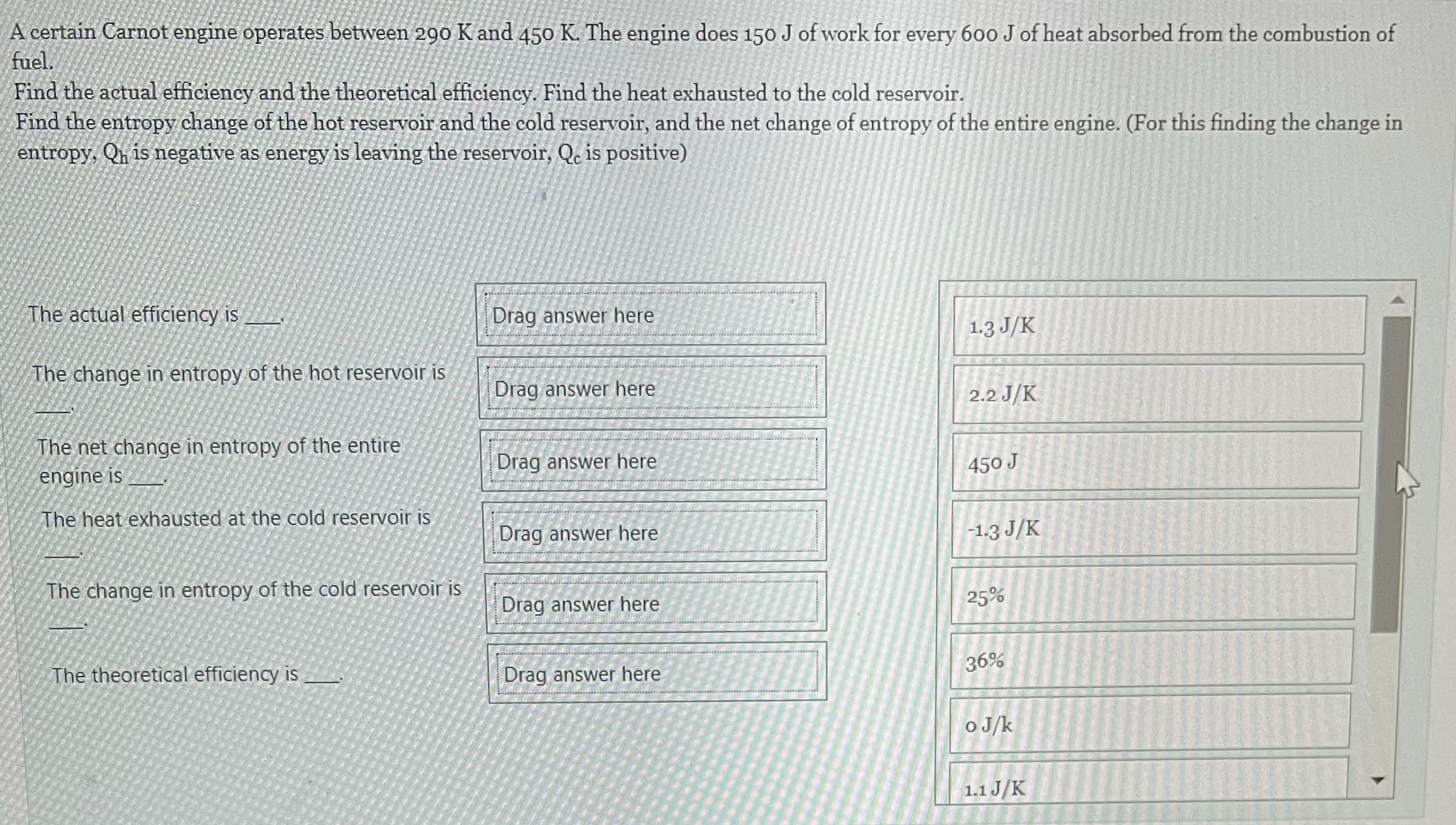
A certain Carnot engine operates between 290 K and 450 K. The engine does 150 J of work for every 600 J of heat absorbed from the combustion of fuel. Find the actual efficiency and the theoretical efficiency. Find the heat exhausted to the cold reservoir. Find the entropy change of the hot reservoir and the cold reservoir, and the net change of entropy of the entire engine. (For this finding the change in entropy, Oh is negative as energy is leaving the reservoir, Q is positive) The actual efficiency is Drag answer here 1.3 J/K The change in entropy of the hot reservoir is Drag answer here 2.2 J/K The net change in entropy of the entire engine is Drag answer here 450 J The heat exhausted at the cold reservoir is Drag answer here -1.3 J/K The change in entropy of the cold reservoir is Drag answer here 25% The theoretical efficiency is Drag answer here 36% o Jk 1.1 J/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


