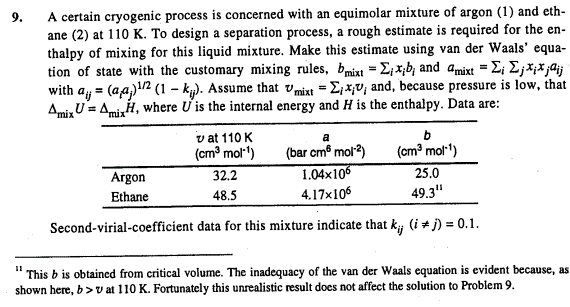
Question: A certain cryogenic process is concerned with an equimolar mixture of argon ( 1 ) and eth - ane ( 2 ) at 1 1
A certain cryogenic process is concerned with an equimolar mixture of argon and eth
ane at To design a separation process, a rough estimate is required for the en
thalpy of mixing for this liquid mixture. Make this estimate using van der Waals' equa
tion of state with the customary mixing rules, and
with Assume that and, because pressure is low, that
where is the internal energy and is the enthalpy. Data are:
Secondvirialcoefficient data for this mixture indicate that
"This is obtained from critical volume. The inadequacy of the van der Waals equation is evident because, as
shown here, at Fortunately this unrealistic result does not affect the solution to Problem
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


