Question: A certain first-order reaction (A products) has a rate constant of 6.90103s1 at 45C. How many minutes does it take for the concentration of the
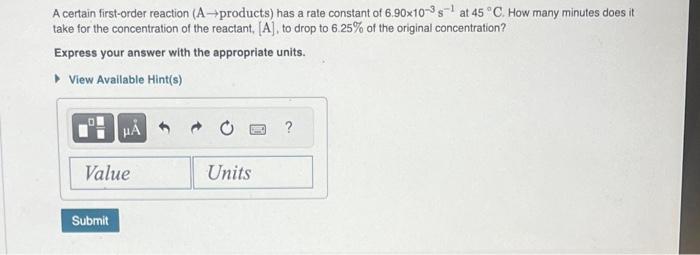
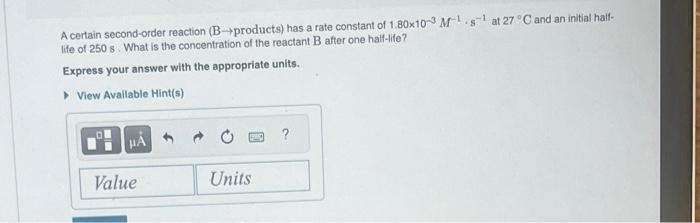
A certain first-order reaction (A products) has a rate constant of 6.90103s1 at 45C. How many minutes does it take for the concentration of the reactant. [A], to drop to 6.25% of the original concentration? Express your answer with the appropriate units. A certain second-order reaction (B products) has a rate constant of 1.80103M1s1 at 27C and an initial halllife of 250s. What is the concentration of the reactant B after one hall-lite? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


