Question: A chemistry student must write down in her la notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the
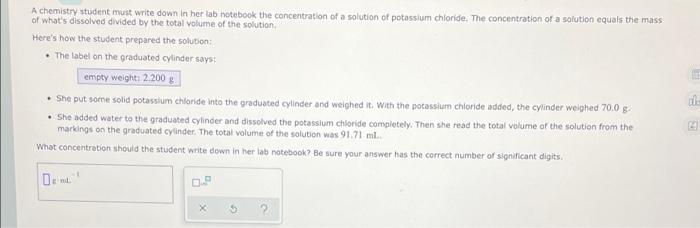
A chemistry student must write down in her la notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution Here's how the student prepared the solution: The label on the graduated cylinder says: empty welght: 2200 g She put some solid potassium chloride into the graduated cylinder and weighed it. With the potassium chloride added, the cylinder weighed 70,0 g She added water to the graduated cylinder and dissolved the potassium chloride completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 9171 m. What concentration should the student write down in her tab notebook? Be sure your answer has the correct number of significant digits. ch 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


