Question: A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the
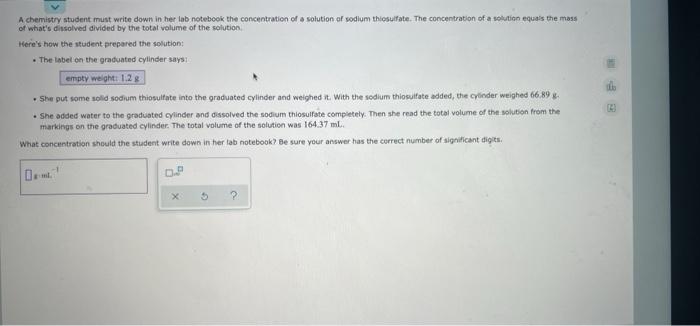
A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution Here's how the student prepared the solution: . The label on the graduated cylinder says empty weight: 1.28 . She put some solid sodium thiosulfate into the graduated cylinder and weighed it with the sodium thiosulfate added, the cylinder weighed 66,898 . She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 16437 ml. What concentration should the student write down in her tab notebook? Be sure your answer has the correct number of significant digits D. 0P X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


