Question: A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments
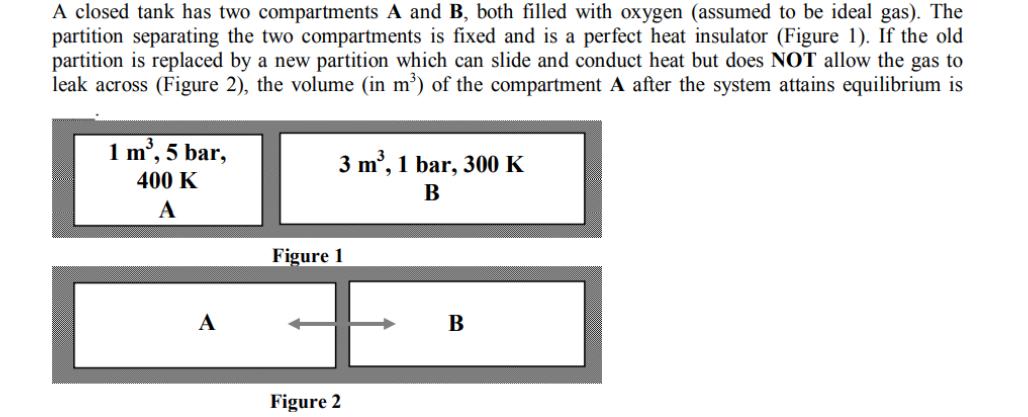
A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure 1). If the old partition is replaced by a new partition which can slide and conduct heat but does NOT allow the gas to leak across (Figure 2), the volume (in m) of the compartment A after the system attains equilibrium is 1 m, 5 bar, 400 K A A 3 m, 1 bar, 300 K B Figure 1 Figure 2 B
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


