Question: A coal sample from Sarawak has an ultimate analysis by mass as shown in TABLE Q4. The coal is burned with 40% access air.
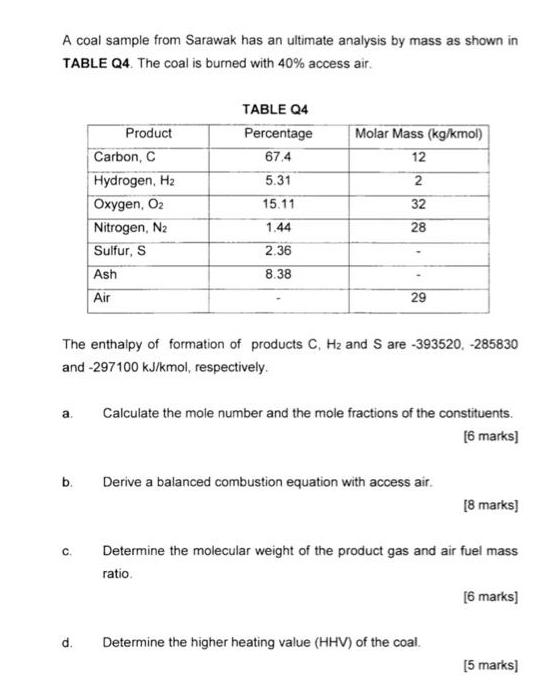
A coal sample from Sarawak has an ultimate analysis by mass as shown in TABLE Q4. The coal is burned with 40% access air. a. b. C. Product d. Carbon, C Hydrogen, H Oxygen, O Nitrogen, N Sulfur, S Ash Air The enthalpy of formation of products C, H and S are -393520, -285830 and -297100 kJ/kmol, respectively. TABLE Q4 Percentage 67.4 5.31 15.11 1.44 2.36 8.38 Molar Mass (kg/kmol) 12 2 32 28 29 Calculate the mole number and the mole fractions of the constituents. [6 marks] Derive a balanced combustion equation with access air. [8 marks] Determine the molecular weight of the product gas and air fuel mass ratio. Determine the higher heating value (HHV) of the coal. [6 marks] [5 marks]
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
a To calculate the mole number and mole fractions of the constituents we need to convert the given percentages to mole fractions First lets calculate the mass of each constituent based on the given pe... View full answer

Get step-by-step solutions from verified subject matter experts


