Question: (a) Colcuiate AS tm( in J/K ) for the dissolution of beryitium fluoride in water. BeF2(a)Be2+(aq)+2F(aq) (b) The third law of thermodynamics states that for
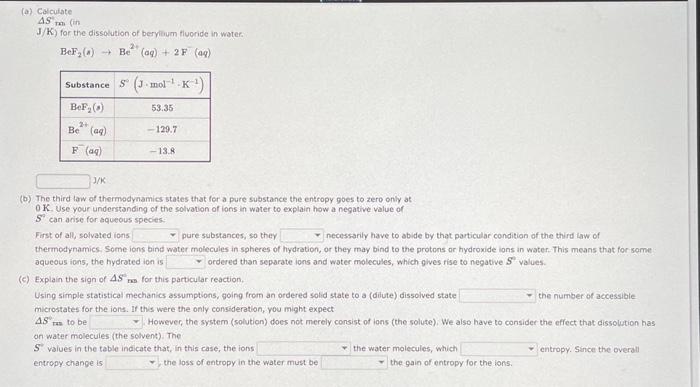
(a) Colcuiate AS tm( in J/K ) for the dissolution of beryitium fluoride in water. BeF2(a)Be2+(aq)+2F(aq) (b) The third law of thermodynamics states that for a pure substance the entropy goes to zero only at 0K. Use your understanding of the solvation of ions in water to explain how a negative value of S? can arise for squeous species: First of all, solvated ions pure substances, so they necessarily have to abide by that particular condition of the third eaw of thermodynamics. Some ions bind water molecules in spheres of hydration, or they may bind to the protons or hydroxide ions in water. This means that for some aqueous ions, the hydrated ion is ordered than separate ions and water molecules, which gives rise to negative S values. (c) Explain the sign of S2 ns for this particular reaction. Using simple statistical mechanics assumptions, going from an ordered solid state to a (dilute) dissolved state the number of accessible microstates for the ions. it this were the only consideration, you might expect S6 tas to be However, the system (solution) does not merely consist of ions (the solute). We also have to consider the effect that dissolution has on water molecuies (the solvent), The S values in the table indicate that, in this case, the ions the water molecules, which entropy. Since the overall entropy change is the loss of entropy in the water must be the gain of entropy for the ions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


