Question: A company is considering converting a gas burner currently using type A fuel to type B fuel). The fuel properties are given below; Molar composition;
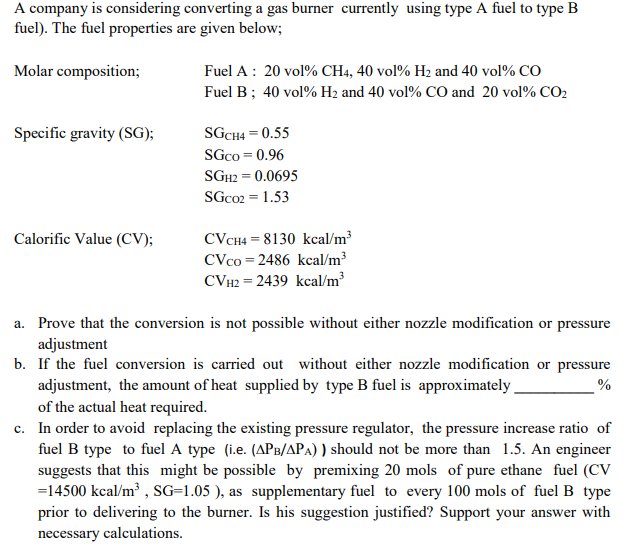
A company is considering converting a gas burner currently using type A fuel to type B fuel). The fuel properties are given below; Molar composition; Fuel A : 20 vol% CH4, 40 vol% H2 and 40 vol% CO Fuel B; 40 vol% H2 and 40 vol% CO and 20 vol% CO2 Specific gravity (SG); SGCH4 = 0.55 SGco = 0.96 SGH2 = 0.0695 SGCO2 = 1.53 Calorific Value (CV); CVCH4 = 8130 kcal/m CV co = 2486 kcal/m CVH2 = 2439 kcal/m a. Prove that the conversion is not possible without either nozzle modification or pressure adjustment b. If the fuel conversion is carried out without either nozzle modification or pressure adjustment, the amount of heat supplied by type B fuel is approximately % of the actual heat required. c. In order to avoid replacing the existing pressure regulator, the pressure increase ratio of fuel B type to fuel A type (i.e. (APB/APA) ) should not be more than 1.5. An engineer suggests that this might be possible by premixing 20 mols of pure ethane fuel (CV =14500 kcal/m , SG=1.05 ), as supplementary fuel to every 100 mols of fuel B type prior to delivering to the burner. Is his suggestion justified? Support your answer with necessary calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


