Question: A compound initially in the solid phase is heated at constant pressure. The enthalpy of vaporization for this compound is greater than its enthalpy of
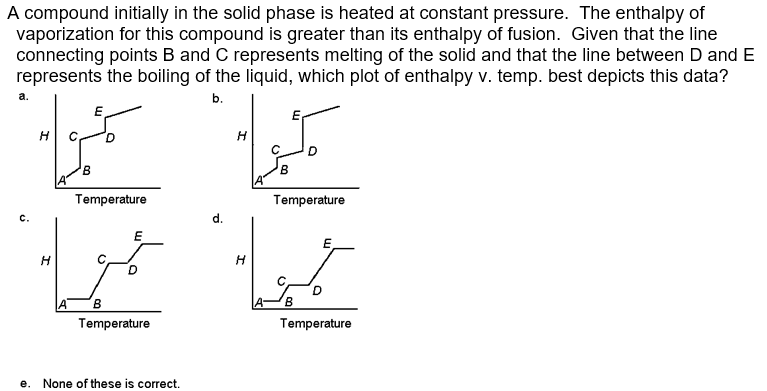
A compound initially in the solid phase is heated at constant pressure. The enthalpy of vaporization for this compound is greater than its enthalpy of fusion. Given that the line connecting points B and C represents melting of the solid and that the line between D and E represents the boiling of the liquid, which plot of enthalpy v. temp. best depicts this data? b. c. d. e. None of these is correct
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


