Question: A compound is analyzed and found to be 64.80% C, 13.62% H, and 21,58% O. What is the chemical formula of this compound? Enter
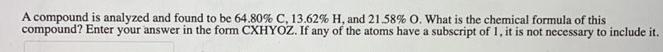
A compound is analyzed and found to be 64.80% C, 13.62% H, and 21,58% O. What is the chemical formula of this compound? Enter your answer in the form CXHYOZ. If any of the atoms have a subscript of 1, it is not necessary to include it.
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Solution Basis 100 g of compound Compounds are assume as 6480 C 1362 H 2158 O Carbon 648 g moles of ... View full answer

Get step-by-step solutions from verified subject matter experts


