Question: A. Consider a simple one component system of n moles with internal energy, U, and volume, V. The entropy, S, and Gibbs potential, G, for
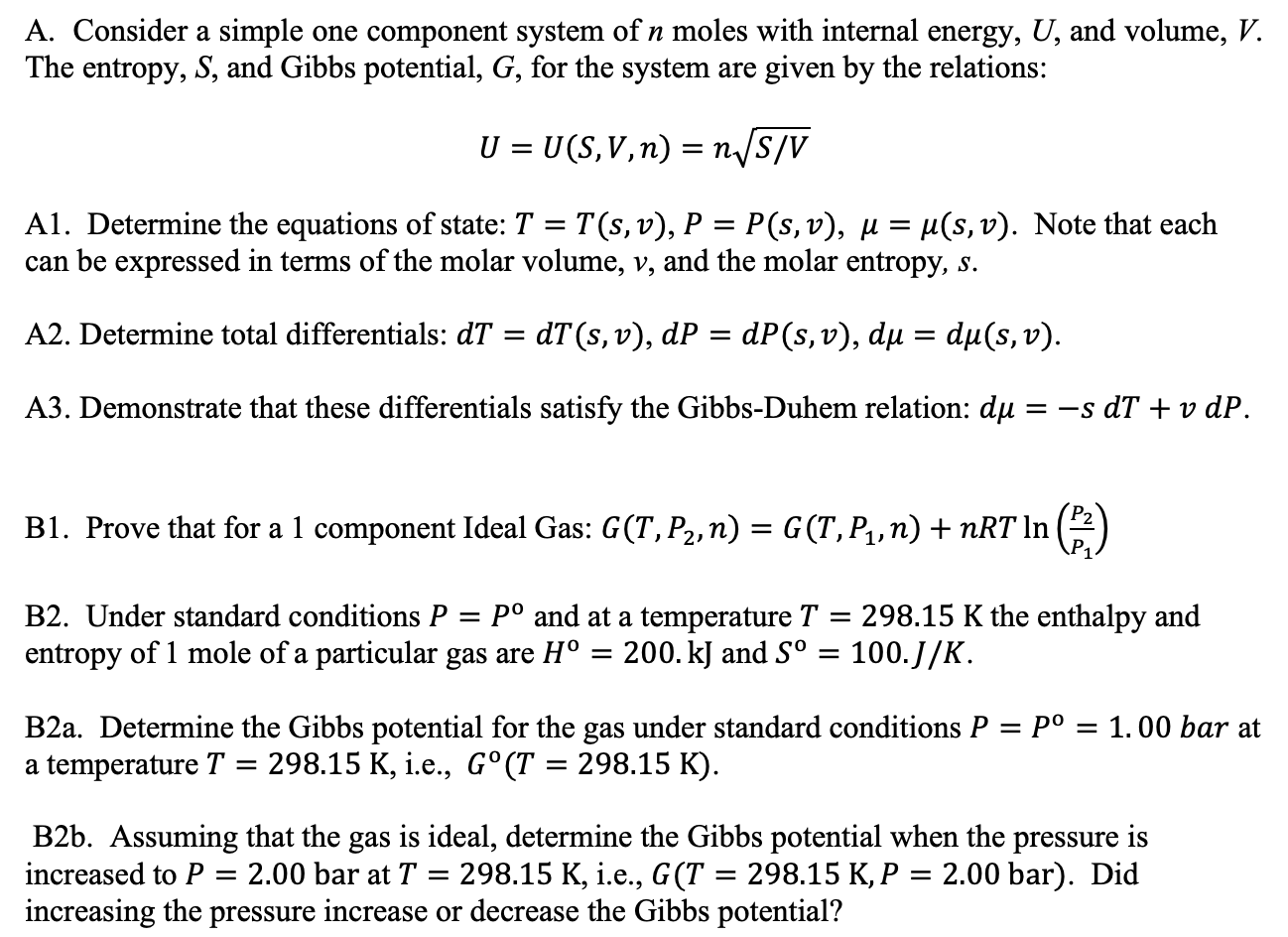
A. Consider a simple one component system of n moles with internal energy, U, and volume, V. The entropy, S, and Gibbs potential, G, for the system are given by the relations: U = U(S, V, n) = n/S/V = Al. Determine the equations of state: T = T(s, v), P P(s, v), u = u(s, v). Note that each can be expressed in terms of the molar volume, v, and the molar entropy, s. A2. Determine total differentials: dT = dT(s, v), dP = dP(s, v), du = du(s, v). = = A3. Demonstrate that these differentials satisfy the Gibbs-Duhem relation: du = -s dT + v dP. = B1. Prove that for a 1 component Ideal Gas: G(T, P2, n) = G(T,P1, n) +nRT In B2. Under standard conditions P p and at a temperature T 298.15 K the enthalpy and entropy of 1 mole of a particular gas are H = 200. kJ and S = 100.J/K. = = B2a. Determine the Gibbs potential for the gas under standard conditions P = p = 1.00 bar at a temperature T = 298.15 K, i.e., G(T = 298.15 K). B2b. Assuming that the gas is ideal, determine the Gibbs potential when the pressure is increased to P = 2.00 bar at T 298.15 K, i.e., G(T = 298.15 K, P = 2.00 bar). Did increasing the pressure increase or decrease the Gibbs potential
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


