Question: a. Consider chemical reaction as shown below: A (9) + 2B (1) - C(g) ..... AH; = -890 kj i. Give the meaning of the
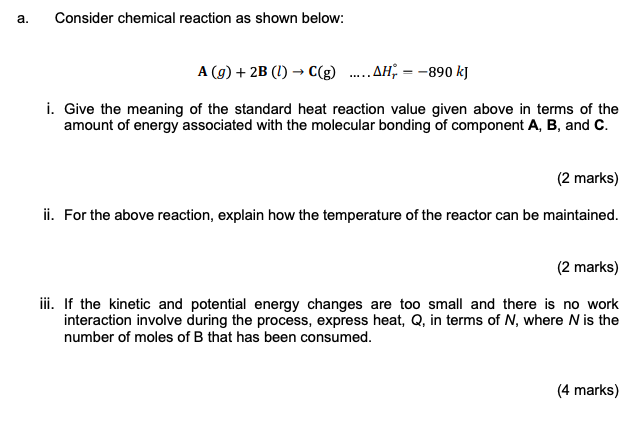
a. Consider chemical reaction as shown below: A (9) + 2B (1) - C(g) ..... AH; = -890 kj i. Give the meaning of the standard heat reaction value given above in terms of the amount of energy associated with the molecular bonding of component A, B, and C. (2 marks) ii. For the above reaction, explain how the temperature of the reactor can be maintained. (2 marks) iii. If the kinetic and potential energy changes are too small and there is no work interaction involve during the process, express heat, Q, in terms of N, where N is the number of moles of B that has been consumed. (4 marks)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


