Question: A continuous fractionating column is to be designed to separate 30,000 kg/h of a mixture of 40 percent benzene and 60 percent toluene into an
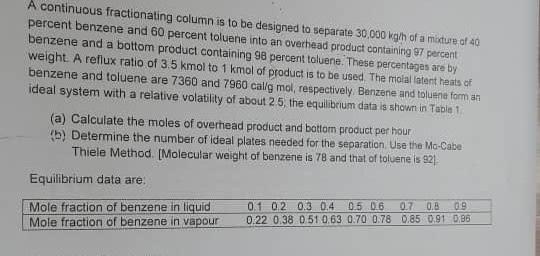
A continuous fractionating column is to be designed to separate 30.000 kg/h of a mixture of 40 percent benzene and 60 percent toluene into an overhead product containing 7 percent benzene and a bottom product containing 98 percent toluene. These percentages are by weight. A reflux ratio of 3.5 kmol to 1 kmol of product is to be used. The molal latent heats of benzene and toluene are 7360 and 7960 calig mol, respectively Benzene and toluene for an ideal system with a relative volatility of about 25, the equilibrium data is shown in Tapie 1 (a) Calculate the moles of overhead product and bottom product per hour (5) Determine the number of ideal plates needed for the separation Use the Mo-Cabe Thiele Method. [Molecular weight of benzene is 78 and that of toluere is 921 Equilibrium data are: Mole fraction of benzene in liquid Mole fraction of benzene in vapour 01 02 0.3 0.4 0.5 0.6 070.B 0.9 0.22 0.38 0.51 0.63 0.70 0.78 0.85 0.91 685
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


