Question: a. Convert the condensed formula below into a line-bond drawing. In your drawing, show every bond and atom, and use wedged and dashed bonds as
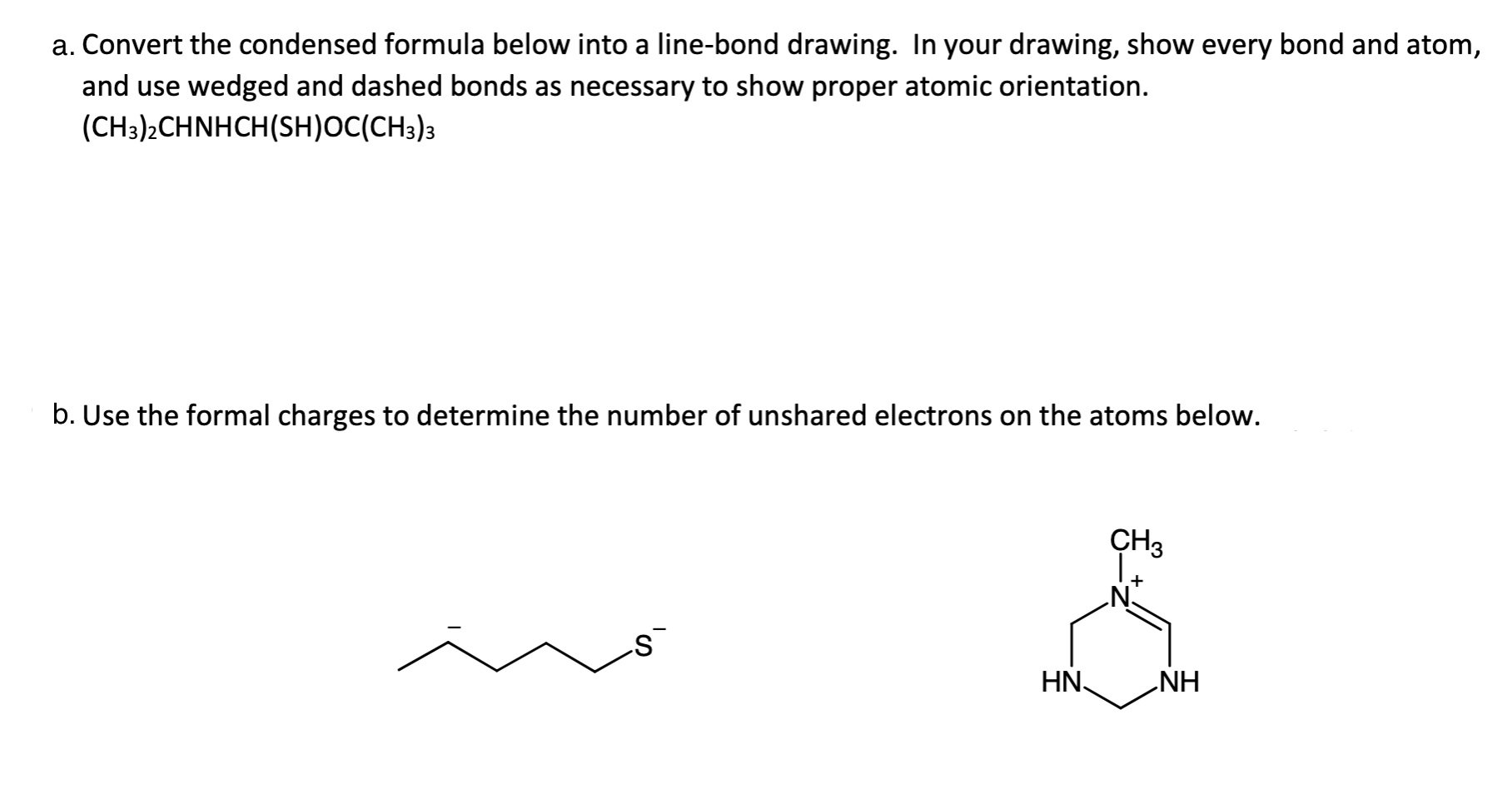
a. Convert the condensed formula below into a line-bond drawing. In your drawing, show every bond and atom, and use wedged and dashed bonds as necessary to show proper atomic orientation. (CH3)2CHNHCH(SH)OC(CH3)3 b. Use the formal charges to determine the number of unshared electrons on the atoms below. CH3 + s HN NH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


