Question: A current of 5.80A is passed through a Pb(NO3)2 solution. How long, in hours, would this current have to be applied to plate out 6.70g
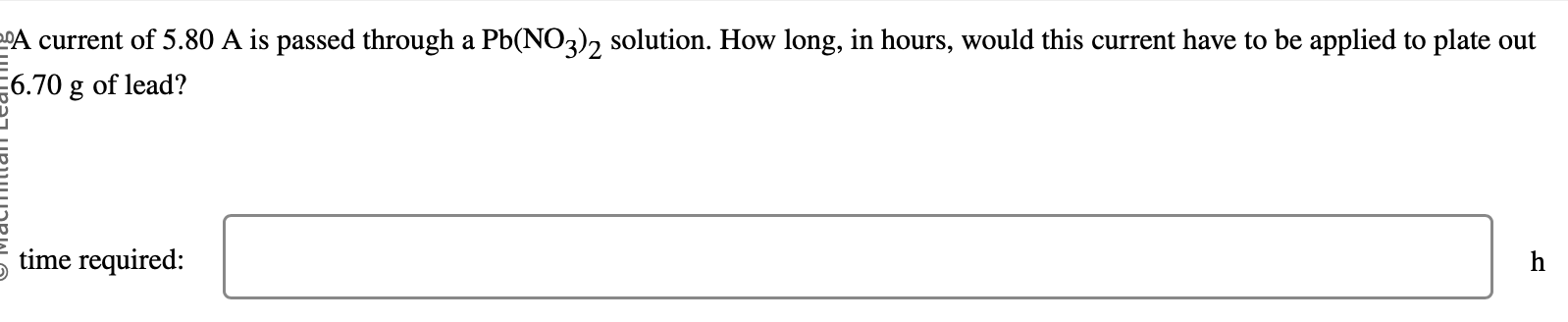
A current of 5.80A is passed through a Pb(NO3)2 solution. How long, in hours, would this current have to be applied to plate out 6.70g of lead
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


