Question: a) Derive the half life equation for a third order reaction 3A Products b) If the third order reaction has an activation energy of 104.6103J/mol
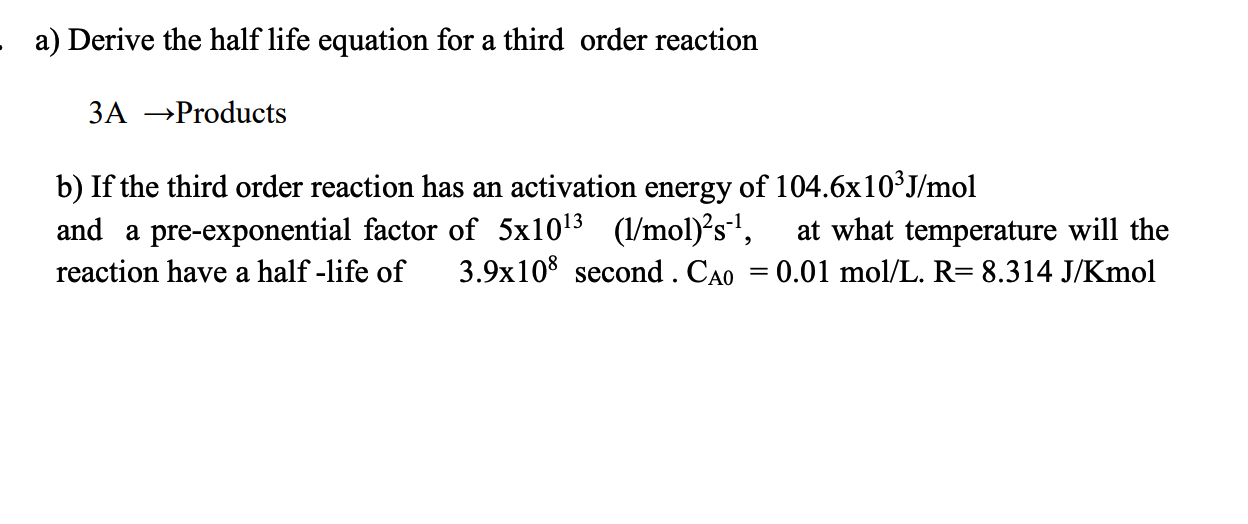
a) Derive the half life equation for a third order reaction 3A Products b) If the third order reaction has an activation energy of 104.6103J/mol and a pre-exponential factor of 51013(1/mol)2s1, at what temperature will the reaction have a half-life of 3.9108 second .CA0=0.01mol/L.R=8.314J/Kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


