Question: A piston-cylinder assembly contains I mol of gaseous ethyl alcohol (CH,OH) and 3.5 mol of oxygen (O) initially at 25 C and I atm.
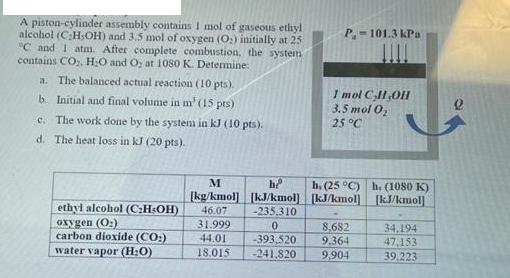
A piston-cylinder assembly contains I mol of gaseous ethyl alcohol (CH,OH) and 3.5 mol of oxygen (O) initially at 25 "C and I atm. After complete combustion, the system contains CO, HO and Oy at 1080 K. Determine: a. The balanced actual reaction (10 pts). b. Initial and final volume in m' (15 pts) c. The work done by the system in kJ (10 pts). d. The heat loss in kJ (20 pts). ethyl alcohol (CH-OH) oxygen (O) carbon dioxide (CO:) water vapor (H:O) P.-101.3 kPa -393,520 -241.820 1 mol C,H,OH 3.5 mol O 25 C M h [kg/kmol] [kJ/kmol] [kJ/kmol] 46.07 -235,310 31.999 0 44.01 18.015 h. (25 C) h. (1080 K) [kJ/kmol 8.682 9.364 9,904 34,194 47,153 39.223 2
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


