Question: a) Develop an expression for lnK as a function of temperature for the following reaction: C6H14+3C2H64CH4+2C4H8 b) The feed to an equilibrium isothermal reactor at
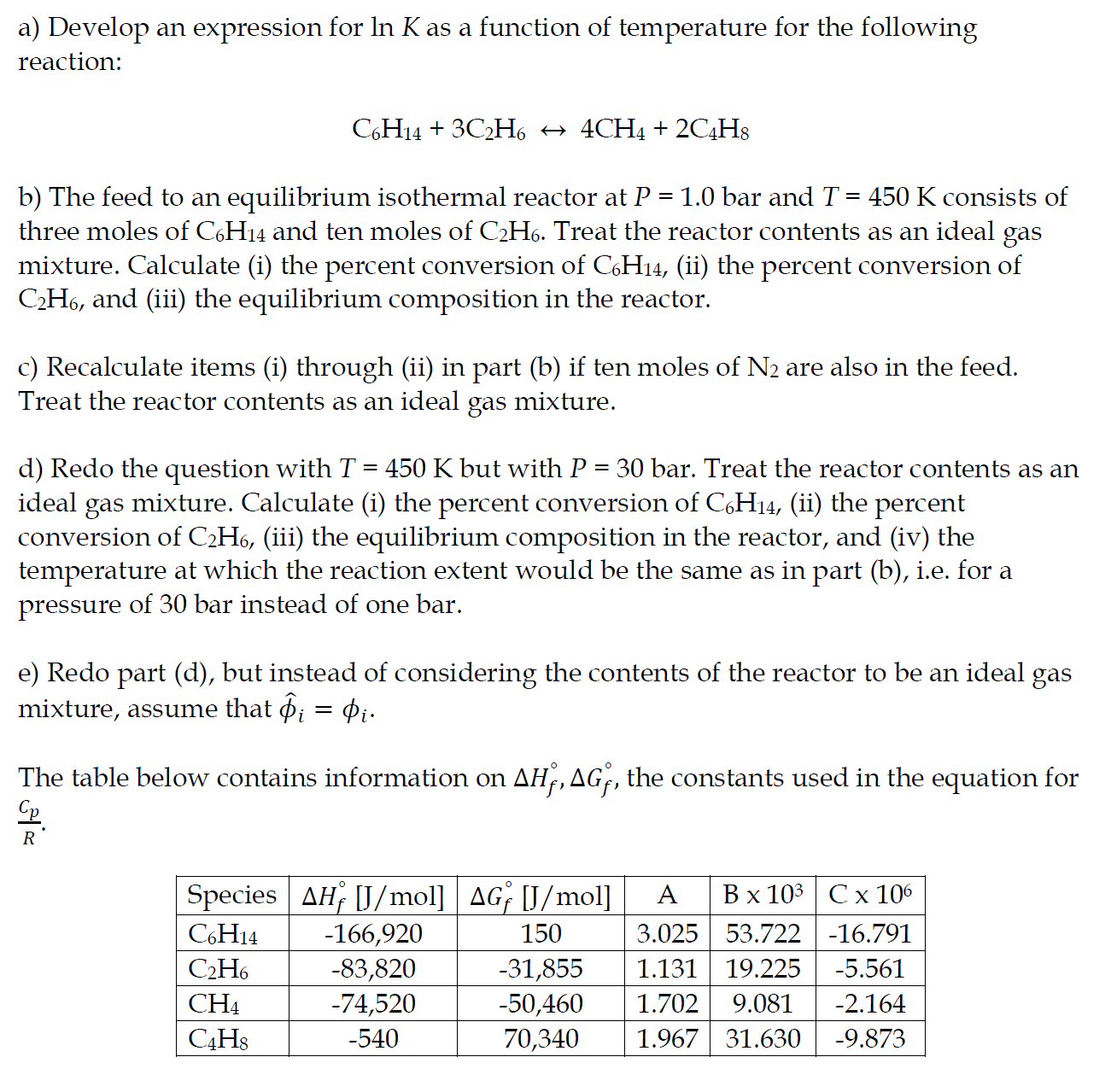
a) Develop an expression for lnK as a function of temperature for the following reaction: C6H14+3C2H64CH4+2C4H8 b) The feed to an equilibrium isothermal reactor at P=1.0 bar and T=450K consists of three moles of C6H14 and ten moles of C2H6. Treat the reactor contents as an ideal gas mixture. Calculate (i) the percent conversion of C6H14, (ii) the percent conversion of C2H6, and (iii) the equilibrium composition in the reactor. c) Recalculate items (i) through (ii) in part (b) if ten moles of N2 are also in the feed. Treat the reactor contents as an ideal gas mixture. d) Redo the question with T=450K but with P=30 bar. Treat the reactor contents as an ideal gas mixture. Calculate (i) the percent conversion of C6H14, (ii) the percent conversion of C2H6, (iii) the equilibrium composition in the reactor, and (iv) the temperature at which the reaction extent would be the same as in part (b), i.e. for a pressure of 30 bar instead of one bar. e) Redo part (d), but instead of considering the contents of the reactor to be an ideal gas mixture, assume that ^i=i. The table below contains information on Hf,Gf, the constants used in the equation for RCp
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


