Question: A. Develop an expression that could be evaluated using PVT EOS for ap (Hint: expression that should only contain measured parameters: P. v, T) B.
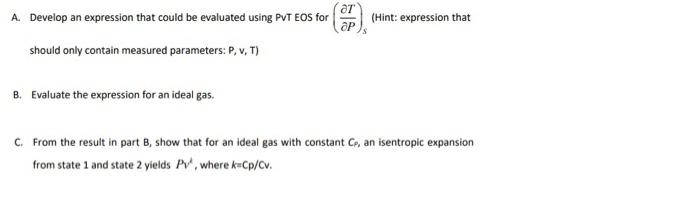

A. Develop an expression that could be evaluated using PVT EOS for ap (Hint: expression that should only contain measured parameters: P. v, T) B. Evaluate the expression for an ideal gas. C. From the result in part B, show that for an ideal gas with constant Co, an isentropic expansion from state 1 and state 2 yields PV, where k=cp/cv. D. Evaluate the expression for a gas that obeys the van der Waals equation of state
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


