Question: a) Does gold diffuse into aluminum via interstitials or substitution? b) According to the Hume-Rothery rules, which metal(s) would most readily form a solid solution
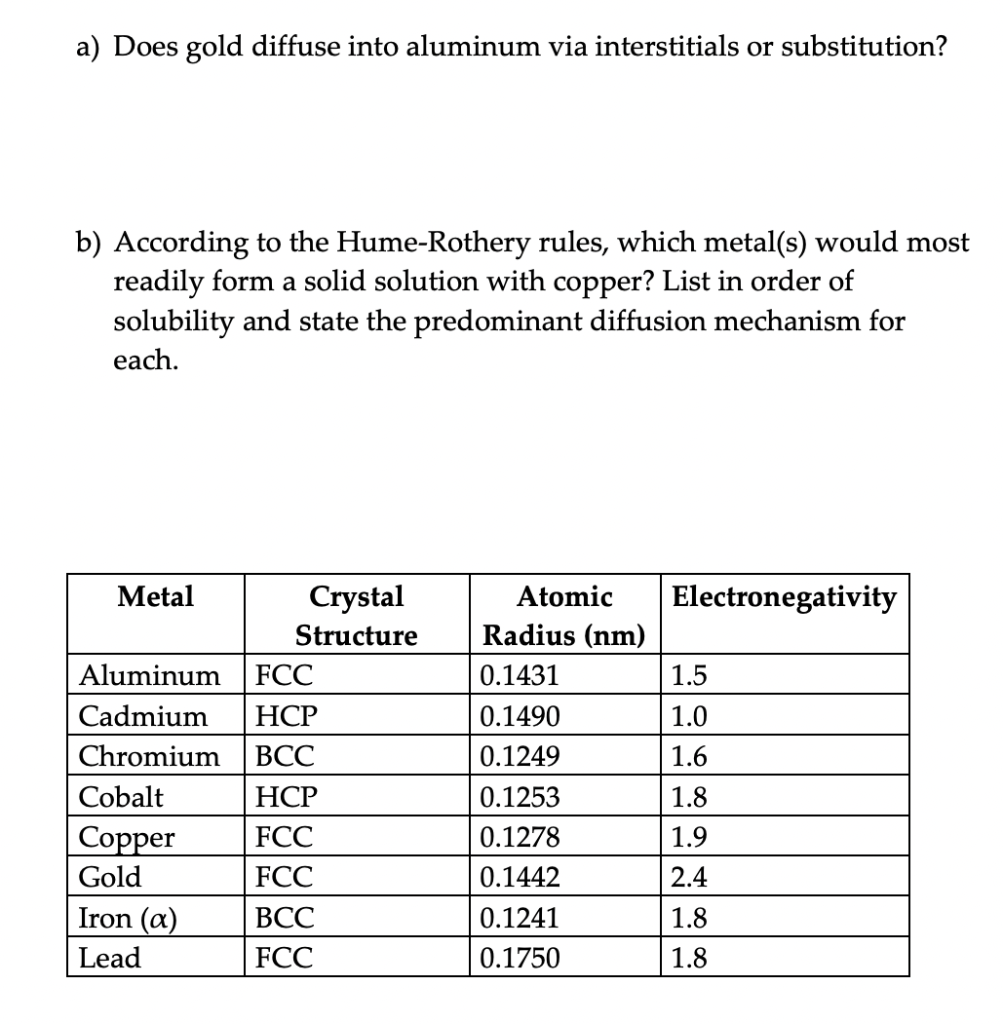
a) Does gold diffuse into aluminum via interstitials or substitution? b) According to the Hume-Rothery rules, which metal(s) would most readily form a solid solution with copper? List in order of solubility and state the predominant diffusion mechanism for each. Electronegativity 1.5 Metal Crystal Structure Aluminum FCC Cadmium HCP Chromium BCC Cobalt HCP Copper FCC Gold FCC BCC Lead FCC Atomic Radius (nm) 0.1431 0.1490 0.1249 0.1253 0.1278 0.1442 1.0 1.6 1.8 1.9 2.4 Iron (a) 0.1241 1.8 0.1750 1.8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


