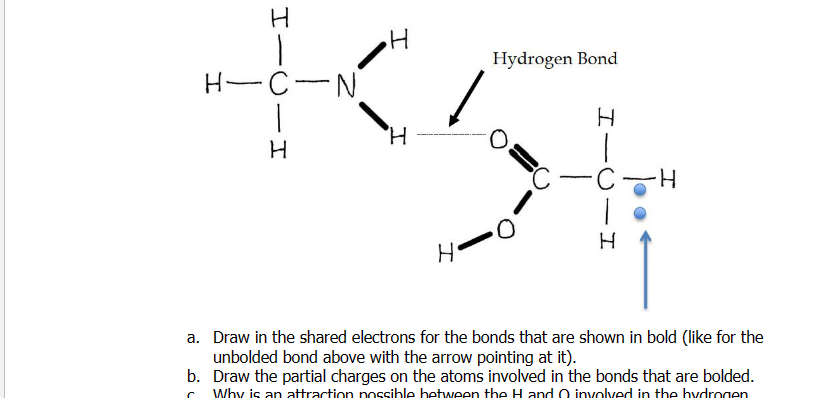
Question: a . Draw in the shared electrons forDraw in the shared electrons for the bonds that are shown in bold ( like for the unbolded
a Draw in the shared electrons forDraw in the shared electrons for the bonds that are shown in bold like for the
unbolded bond above with the arrow pointing at it
b Draw the partial charges on the atoms involved in the bonds that are bolded.
c Why is an attraction possible between the H and O involved in the hydrogen
bond indicated with the dotted line shown above?
d Draw an alternative hydrogen bond between different atoms of these two
molecules in the space below:
e Go back to Question model and draw in some of the possible hydrogen bonds
in both mixtures.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


