Question: (a) Explain how the graphs indicate that the reaction is second order. (c) Consider two possibite mechanisms for the decomposition reaction. (i) Is the rate
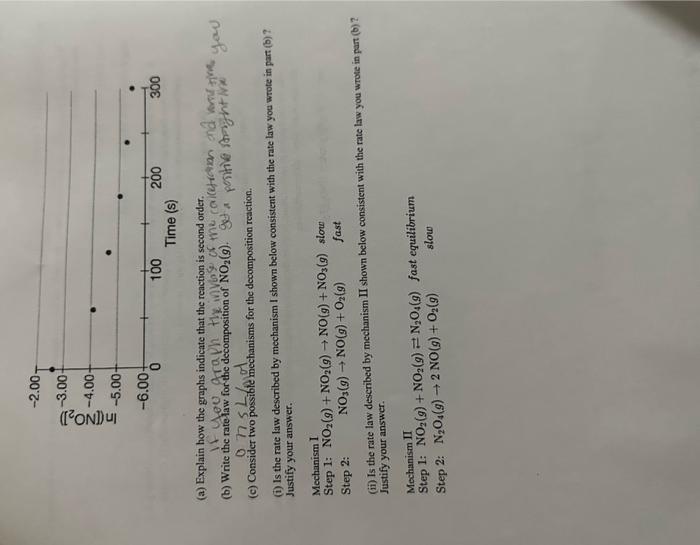
(a) Explain how the graphs indicate that the reaction is second order. (c) Consider two possibite mechanisms for the decomposition reaction. (i) Is the rate law described by mechanism I shown below consistent with the rate law you wrote in part (b) ? Justify your answer. Mechanism I Step 1: NO2(g)+NO2(g)NO(g)+NO3(g) slow Step 2: NO3(g)NO(g)+O2(g) fast (ii) Is the rate law deseribed by mechanism II shown below consistent with the rate law you wrote in part (b) ? Justify your answer. Mechanism II Step 1: NO2(g)+NO2(g)N2O4(g) fast equilibrium Step 2: N2O4(g)2NO(g)+O2(g) slow (a) Explain how the graphs indicate that the reaction is second order. (c) Consider two possibite mechanisms for the decomposition reaction. (i) Is the rate law described by mechanism I shown below consistent with the rate law you wrote in part (b) ? Justify your answer. Mechanism I Step 1: NO2(g)+NO2(g)NO(g)+NO3(g) slow Step 2: NO3(g)NO(g)+O2(g) fast (ii) Is the rate law deseribed by mechanism II shown below consistent with the rate law you wrote in part (b) ? Justify your answer. Mechanism II Step 1: NO2(g)+NO2(g)N2O4(g) fast equilibrium Step 2: N2O4(g)2NO(g)+O2(g) slow
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


