Question: Written assignment 4-Due Wednesday Aug 3rd in class You are allowed to use your notes, but your first attempt should be noteless to see
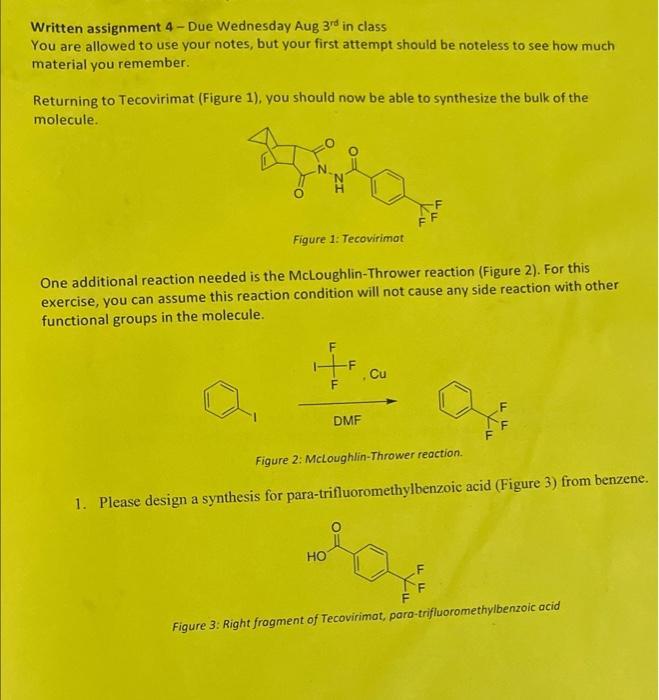
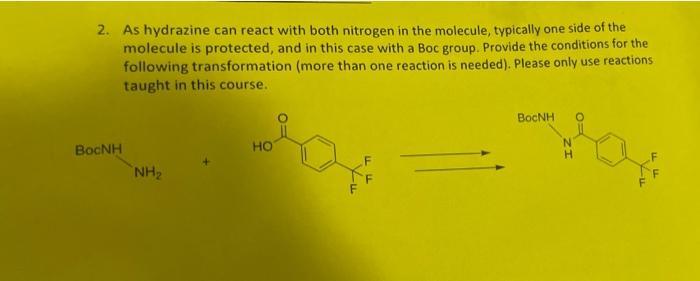
Written assignment 4-Due Wednesday Aug 3rd in class You are allowed to use your notes, but your first attempt should be noteless to see how much material you remember. Returning to Tecovirimat (Figure 1), you should now be able to synthesize the bulk of the molecule. Figure 1: Tecovirimat One additional reaction needed is the McLoughlin-Thrower reaction (Figure 2). For this exercise, you can assume this reaction condition will not cause any side reaction with other functional groups in the molecule. F HO -F DMF Cu Figure 2: McLoughlin-Thrower reaction. 1. Please design a synthesis for para-trifluoromethylbenzoic acid (Figure 3) from benzene. FF FF Figure 3: Right fragment of Tecovirimat, para-trifluoromethylbenzoic acid 2. As hydrazine can react with both nitrogen in the molecule, typically one side of the molecule is protected, and in this case with a Boc group. Provide the conditions for the following transformation (more than one reaction is needed). Please only use reactions taught in this course. Bnh NH HO FF BOCNH N H TI-A LLF
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Question 1 Synthesis of paratrifluoromethylbenzoic acid from benzene Target Molecule paraTrifluoromethylbenzoic acid Synthetic Plan Retrosynthesis Tar... View full answer

Get step-by-step solutions from verified subject matter experts


