Question: A first-order reaction A ---> B occurs in both reactors, with reaction rate constant k. The volumes of liquid in the reactors, V, are constant
A first-order reaction A ---> B occurs in both reactors, with reaction rate constant k. The volumes of liquid in the reactors, V, are constant and equal; the flow rates F0, F1, F2 and R are constant. Assume constant physical properties and a negligible time delay for the recycle line.
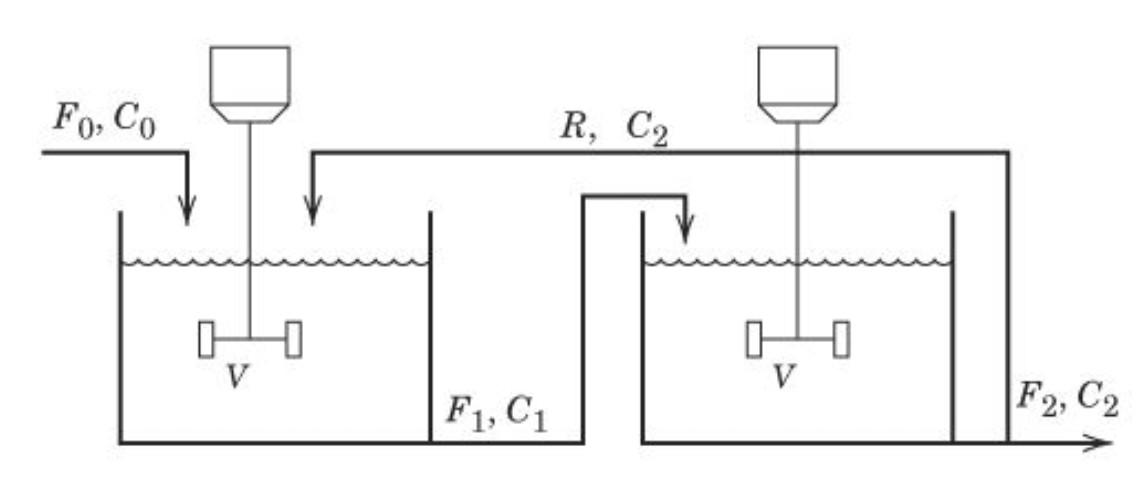
1) Write a mathematical model for this process:
2) Derive a transfer function model relating the output concentration of A, C2 to the inlet concentration of A, C0.
Fo, Co R, C2 1 V V F1, C1 F2, C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


