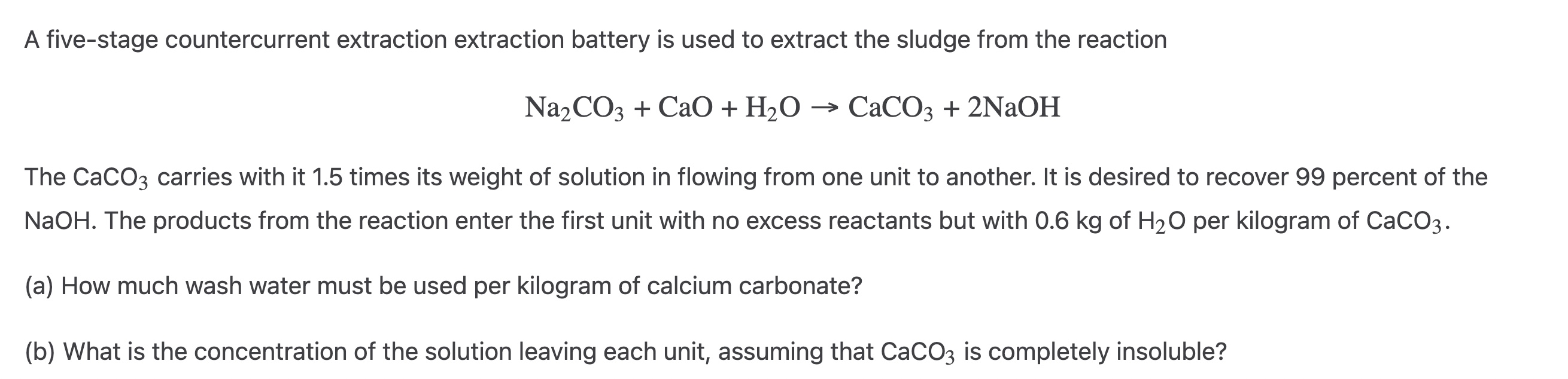
Question: A five - stage countercurrent extraction extraction battery is used to extract the sludge from the reaction N a 2 C O 3 + CaO
A fivestage countercurrent extraction extraction battery is used to extract the sludge from the reaction
CaONaOH
The carries with it times its weight of solution in flowing from one unit to another. It is desired to recover percent of the
NaOH. The products from the reaction enter the first unit with no excess reactants but with of per kilogram of
a How much wash water must be used per kilogram of calcium carbonate?
b What is the concentration of the solution leaving each unit, assuming that is completely insoluble?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


