Question: A flask is charged with 0 . 1 4 0 mol of A and allowed to react to form B according to the following hypothetical
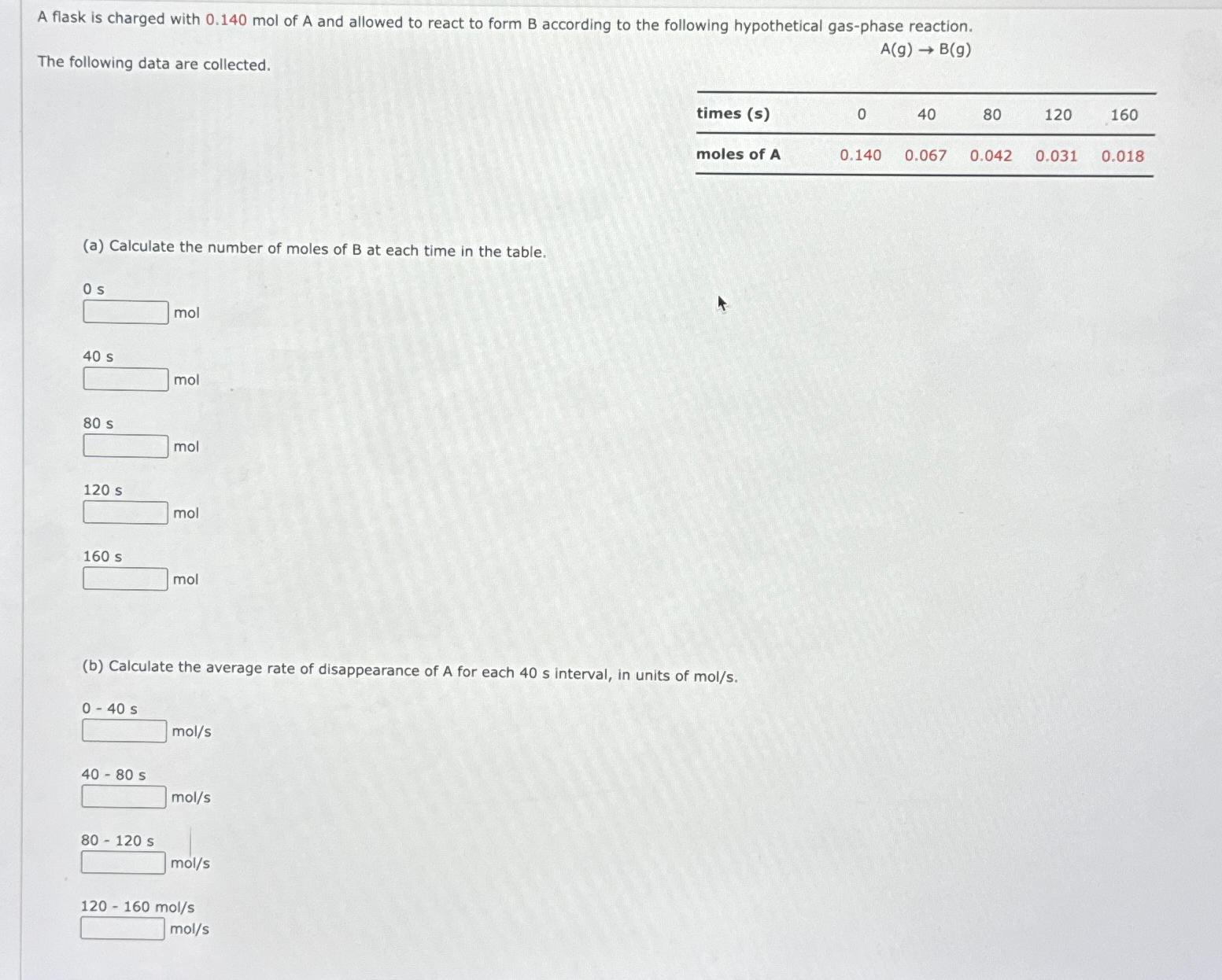
A flask is charged with mol of A and allowed to react to form B according to the following hypothetical gasphase reaction.
The following data are collected.
tabletimes smoles of A
a Calculate the number of moles of B at each time in the table.
s
mol
mol
s
mol
s
mol
s
mol
b Calculate the average rate of disappearance of A for each interval, in units of mols
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


