Question: A flask is filled with 1.56 L (L = liter) of a liquid at 96.2 .C. When the liquid is cooled to 17.8 C, its
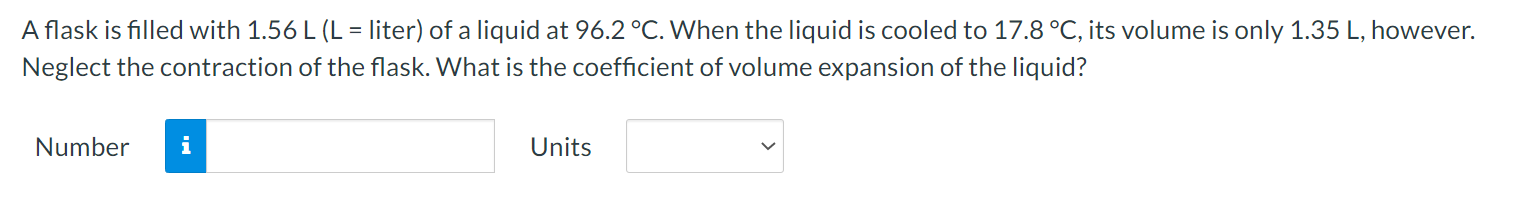
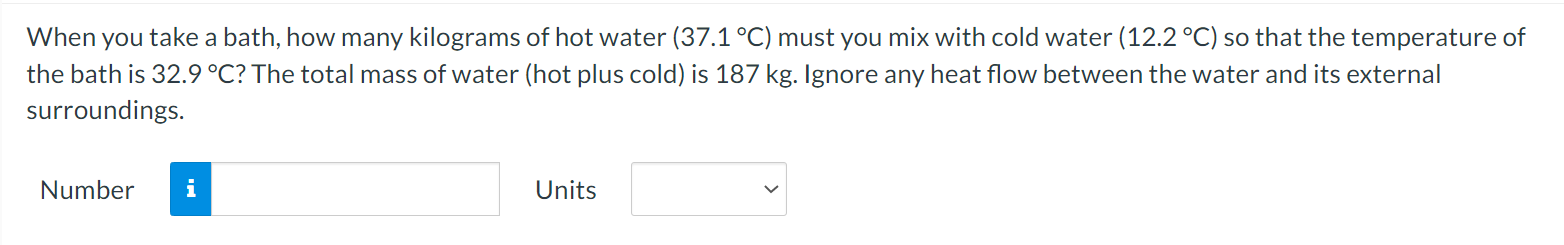
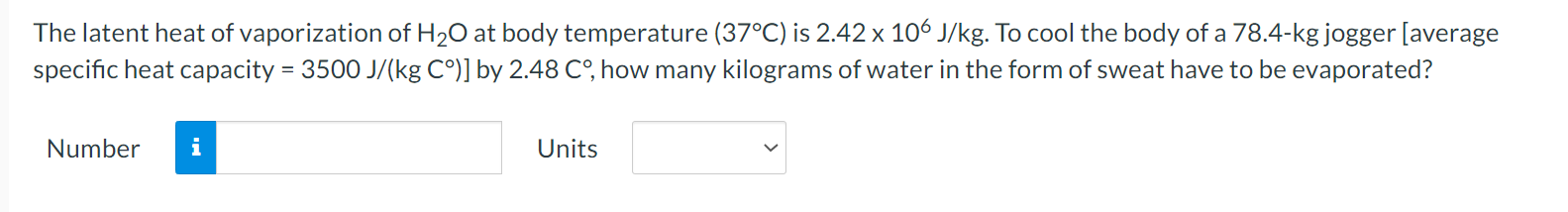
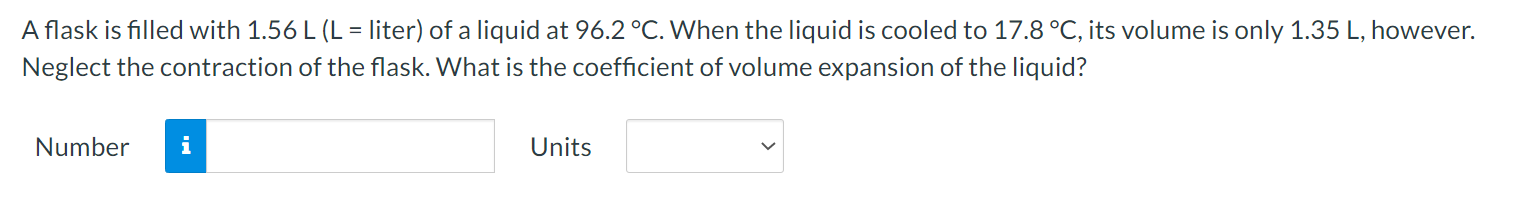
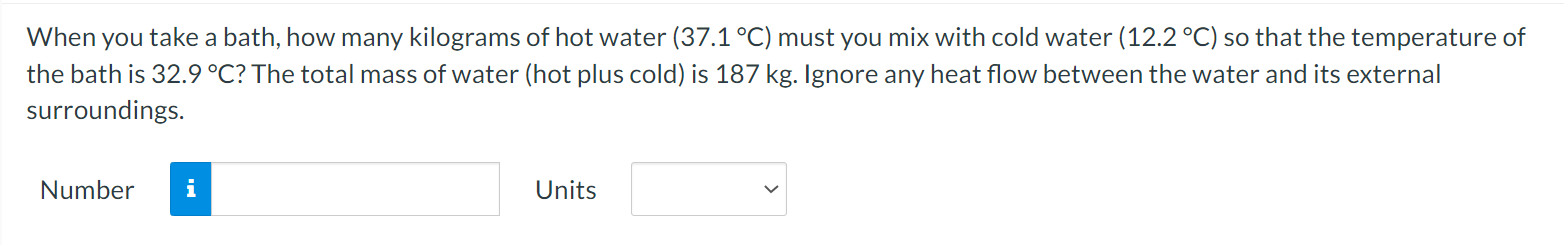
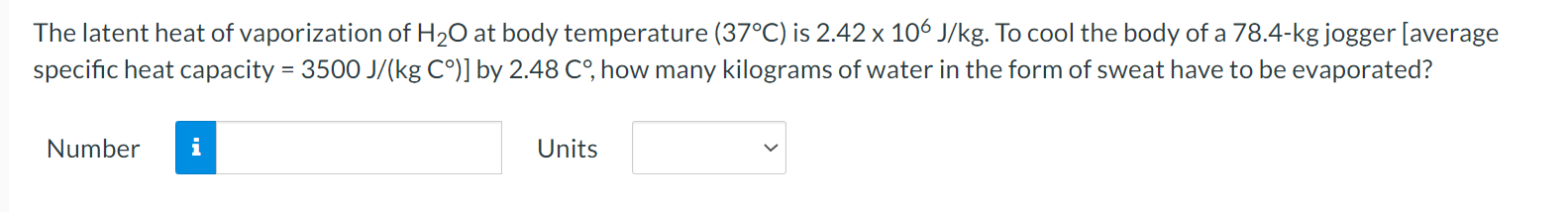
A flask is filled with 1.56 L (L = liter) of a liquid at 96.2 .C. When the liquid is cooled to 17.8 C, its volume is only 1.35 L, however. Neglect the contraction of the flask. What is the coefficient of volume expansion of the liquid? Number i UnitsWhen you take a bath, how many kilograms of hot water (37.1 C) must you mix with cold water (12.2 C) so that the temperature of the bath is 32.9 C? The total mass of water (hot plus cold) is 187 kg. Ignore any heat ow between the water and its external surroundings. Number n Units V The latent heat of vaporization of H20 at body temperature (37C) is 2.42 x 106 J/kg. To cool the body of a 78.4-kgjogger [average specic heat capacity = 3500 J/(kg C)] by 2.48 C, how many kilograms of water in the form of sweat have to be evaporated? Number n Units V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


