Question: A forensic chemist is given a white solid that is suspected of being pure cocaine (C17H21NO4). She dissolves 1.22g of the solid in 15.60g of
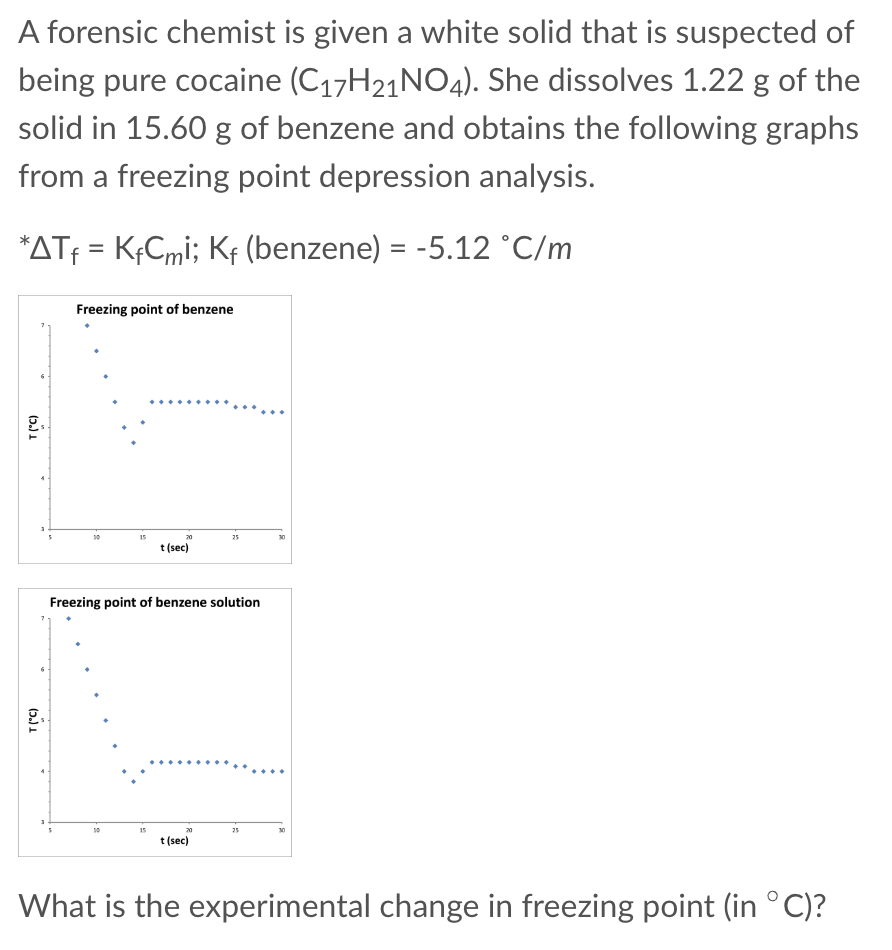



A forensic chemist is given a white solid that is suspected of being pure cocaine (C17H21NO4). She dissolves 1.22g of the solid in 15.60g of benzene and obtains the following graphs from a freezing point depression analysis. Tf=KfCmi;Kf(benzene)=5.12C/m What is the experimental change in freezing point (in C) ? Continuing from question 2 above. For this mass of cocaine (1.22g), what would be the expected change in freezing point (in C )? Continuing from question 2 above. How many moles of solute are required to produce this drop in freezing temperature? Continuing from question 2 above. According to this data, is the substance likely to be cocaine? Yes No More information is needed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


