Question: A fuel cell differs from a battery because the current is being generated from a reduction-oxidation (redox) reaction in which the reactant is consumed. A
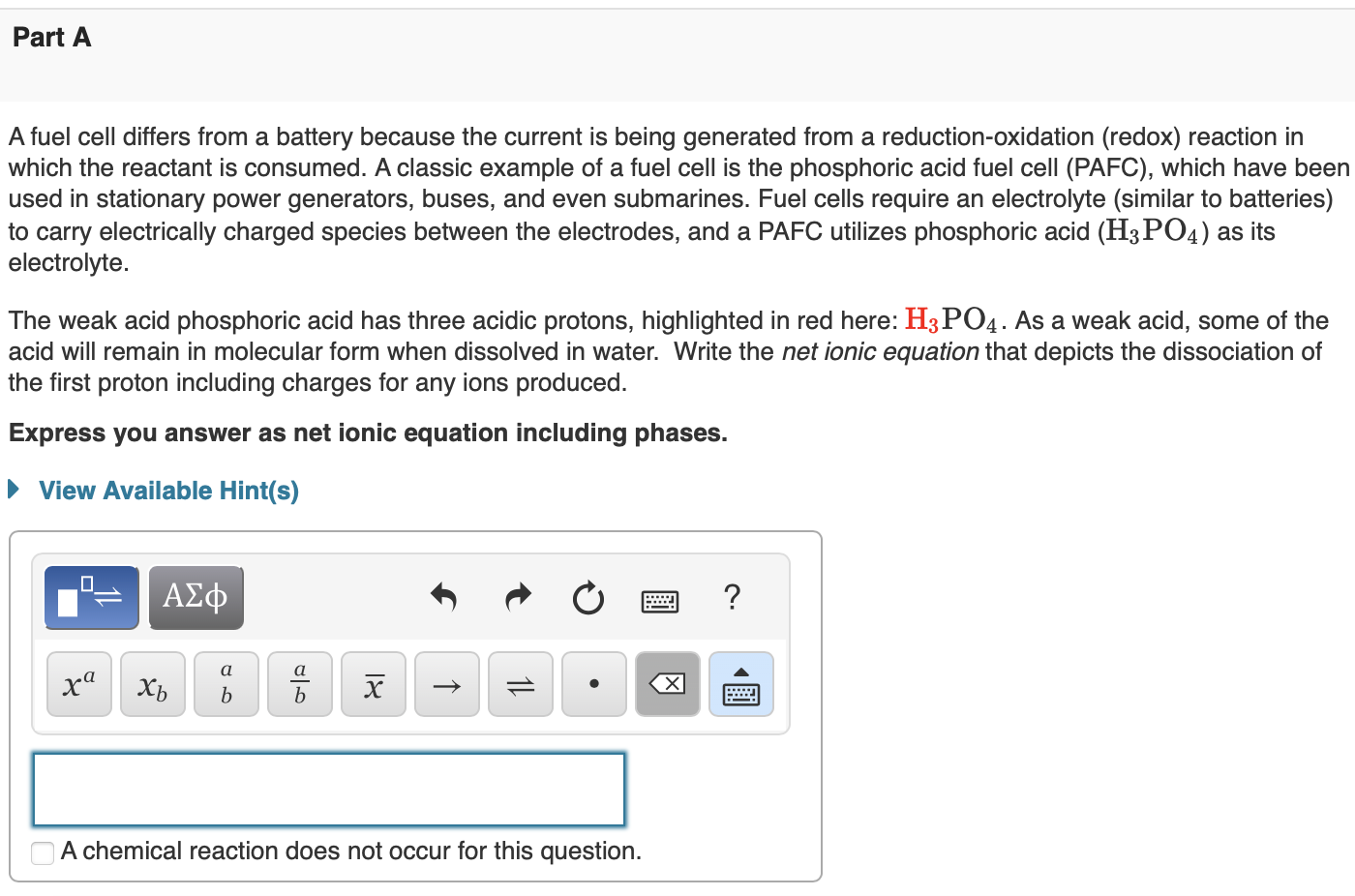
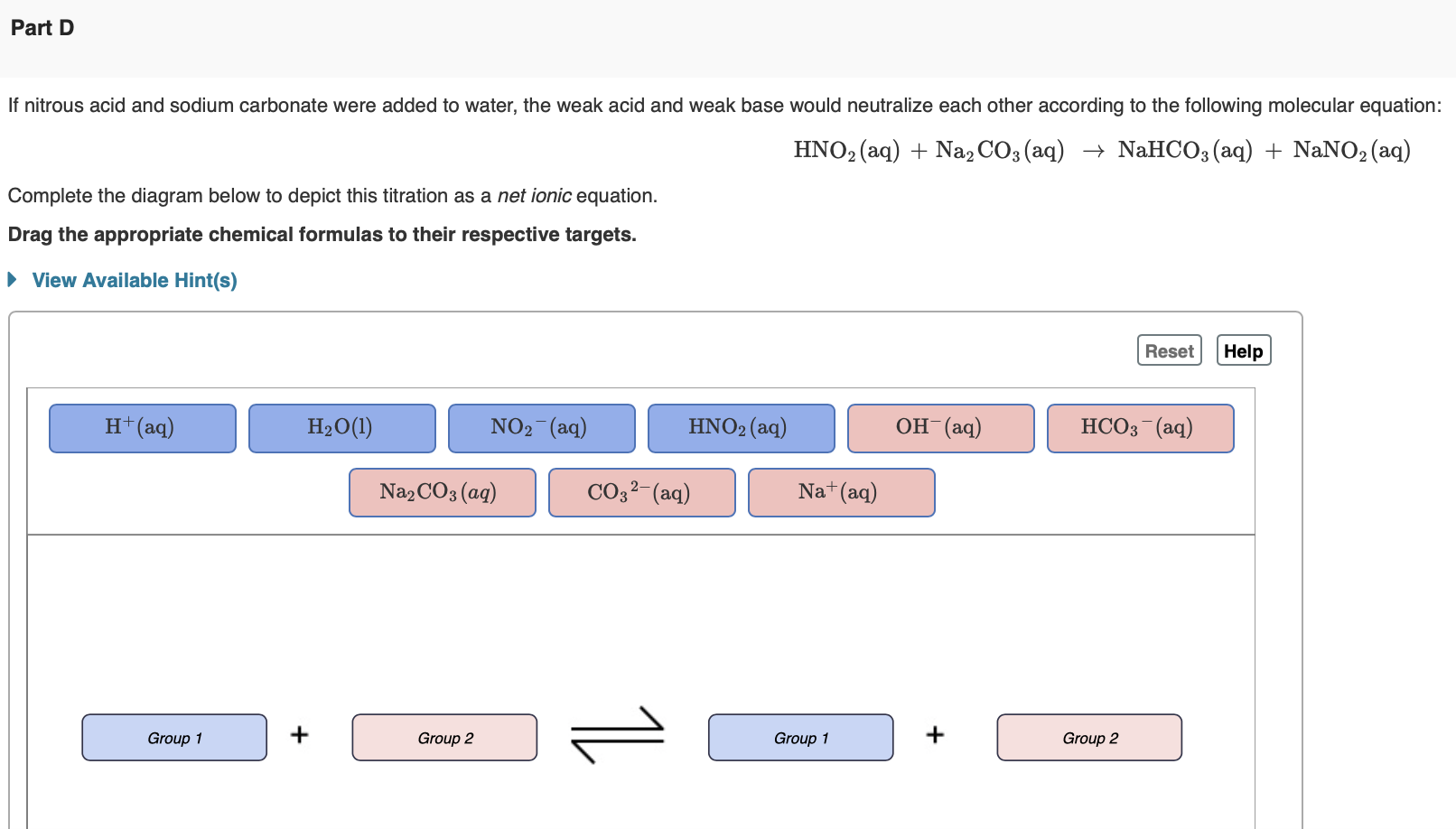
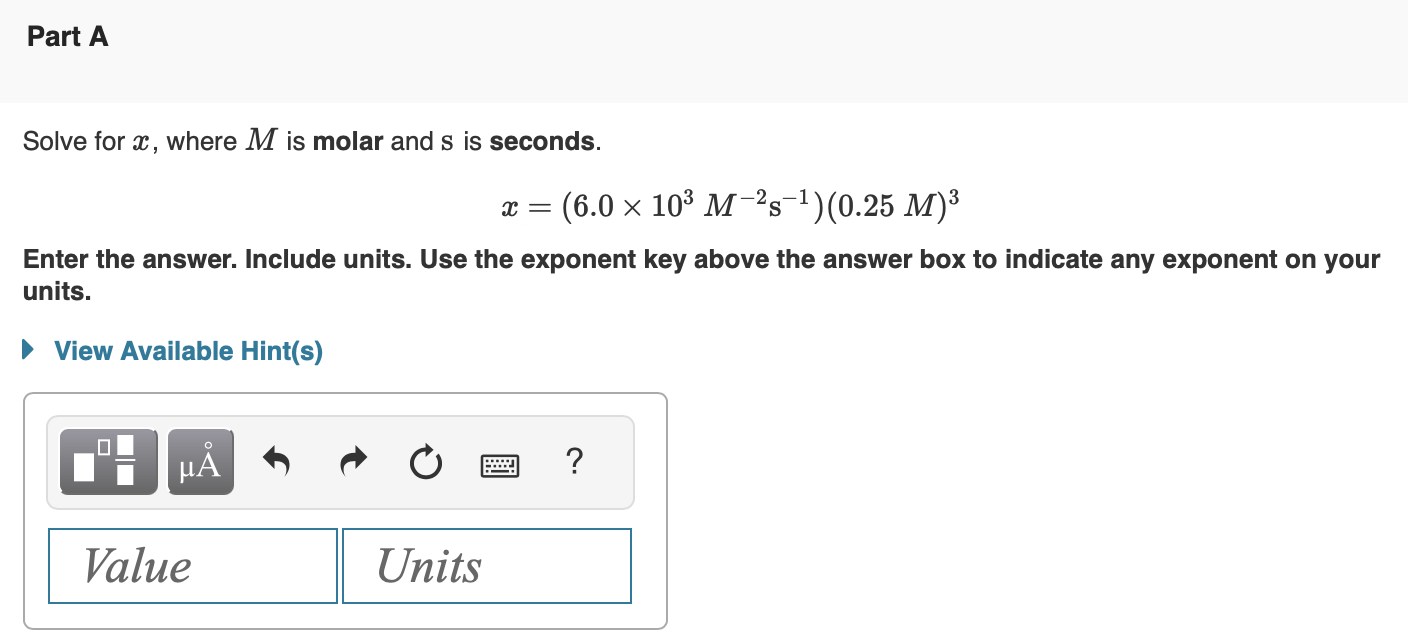
A fuel cell differs from a battery because the current is being generated from a reduction-oxidation (redox) reaction in which the reactant is consumed. A classic example of a fuel cell is the phosphoric acid fuel cell (PAFC), which have been used in stationary power generators, buses, and even submarines. Fuel cells require an electrolyte (similar to batteries) to carry electrically charged species between the electrodes, and a PAFC utilizes phosphoric acid (H3PO4) as its electrolyte. The weak acid phosphoric acid has three acidic protons, highlighted in red here: H3PO4. As a weak acid, some of the acid will remain in molecular form when dissolved in water. Write the net ionic equation that depicts the dissociation of the first proton including charges for any ions produced. Express you answer as net ionic equation including phases. If nitrous acid and sodium carbonate were added to water, the weak acid and weak base would neutralize each other according to the following molecular equation: HNO2(aq)+Na2CO3(aq)NaHCO3(aq)+NaNO2(aq) Complete the diagram below to depict this titration as a net ionic equation. Drag the appropriate chemical formulas to their respective targets. Solve for x, where M is molar and s is seconds. x=(6.0103M2s1)(0.25M)3 Enter the answer. Include units. Use the exponent key above the answer box to indicate any exponent on your units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


