Question: A fuel with the following reported analysis (wt. %) is burnt in an industrial boiler for steam to pasteurize milk. UNSO C H VM
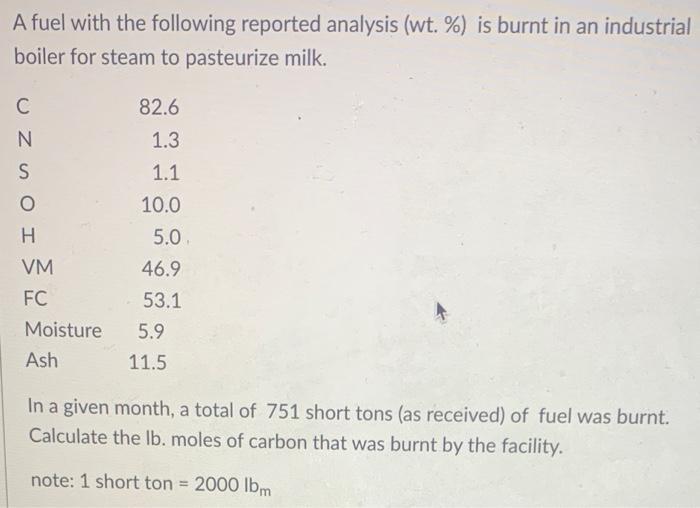

A fuel with the following reported analysis (wt. %) is burnt in an industrial boiler for steam to pasteurize milk. UNSO C H VM FC Moisture Ash 82.6 1.3 1.1 10.0 5.0 46.9 - 53.1 5.9 11.5 In a given month, a total of 751 short tons (as received) of fuel was burnt. Calculate the lb. moles of carbon that was burnt by the facility. note: 1 short ton = 2000 lbm A solid fuel analysis from a chemical lab is shown below (wt.%). Carbon Nitrogen Sulfur 71.2 1 1.3 Oxygen 6.1 Hydrogen 3.9 Moisture 6.1 10.4 38.7 44.8 Ash VM FC What is the dry hydrogen (wt.%). Round the answer to one decimal place.
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Q1 Solution Given 512 short tons of fuel was burnt 512 x 2000 1024000 ... View full answer

Get step-by-step solutions from verified subject matter experts


