Question: A gas is in a container with a piston lid and is taken from thermodynamic state, 1, to a new thermo- dynamic state, 2, by
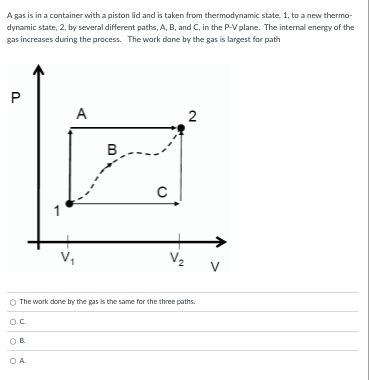
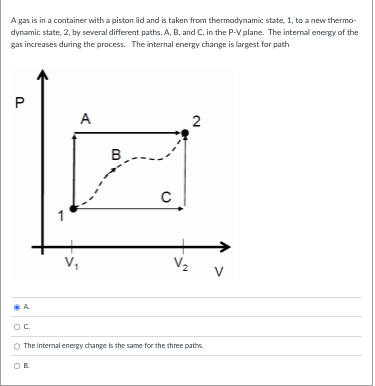
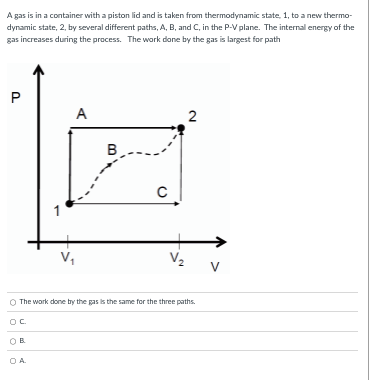
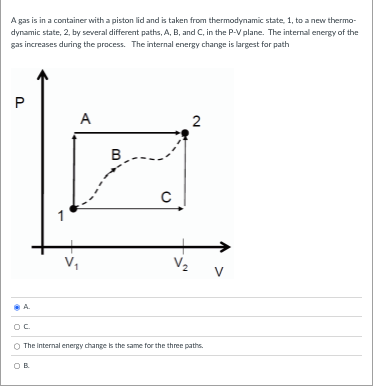
A gas is in a container with a piston lid and is taken from thermodynamic state, 1, to a new thermo- dynamic state, 2, by several different paths, A, B, and C, in the P-V plane. The internal energy of the gas increases during the process. The work done by the gas is largest for path P A 2 B C 1 V. V 2 V The work done by the pas Is the same for the three paths. OC OB O A.A gas is in a container with a piston lid and is taken from thermodynamic state, 1, to a new thermo- dynamic state, 2, by several different paths, A, B, and C, in the P-V plane. The internal energy of the gas increases during the process. The internal energy change is largest for path P A 2 B C V V 2 V A The Internal energy change is the same for the three paths
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


