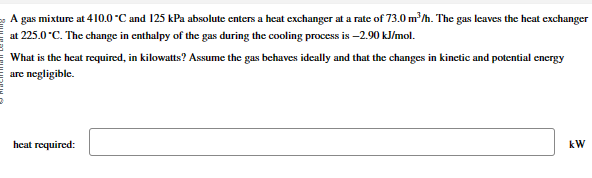
Question: A gas mixture at 4 1 0 . 0 - C and 1 2 5 kPa absolute enters a heat exchanger at a rate of
A gas mixture at and kPa absolute enters a heat exchanger at a rate of The gas leaves the heat exchanger
at The change in enthalpy of the gas during the cooling process is
What is the heat required, in kilowatts? Assume the gas behaves ideally and that the changes in kinetic and potential energy
are negligible.
heat required:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


