Question: A gaseous fuel mixture contains 2 4 1 % methane ( C H 4 ) , 3 0 7 % ethane ( C 2 H
A gaseous fuel mixture contains methane ethane and the rest propane by volume
Part A
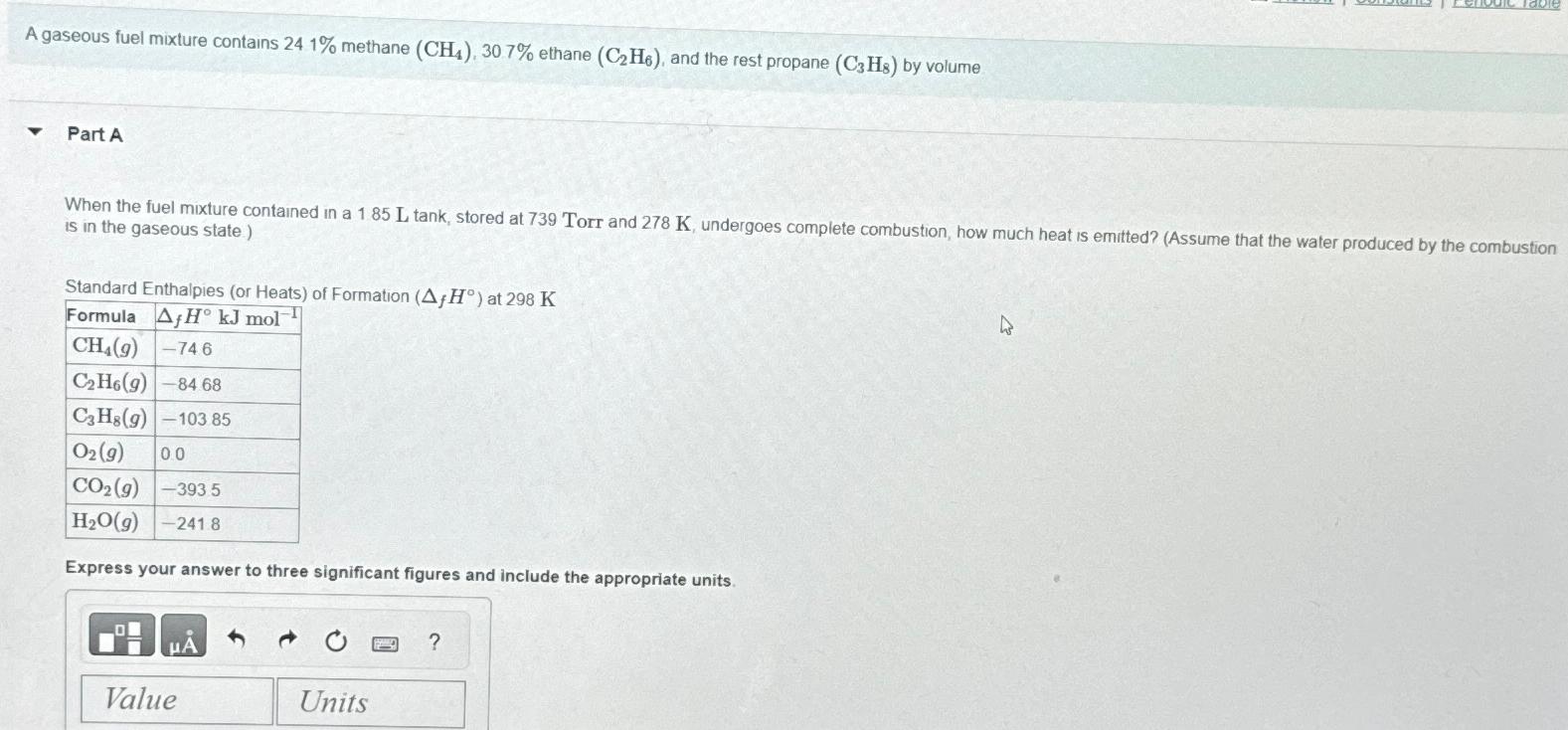
When the fuel mixture contained in a tank stored at Torr and undergoes complete combustion, how much heat is emited? Assume that the water produced by the combustion is in the gaseous state
Standard Enthalpies or Heats of Formation at
tableFormula
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


