Question: A gasoline engine uses 2 moles of monoatomic ideal gas as the working substance. Figure 3 shows the thermodynamic cycle taken by the engine.
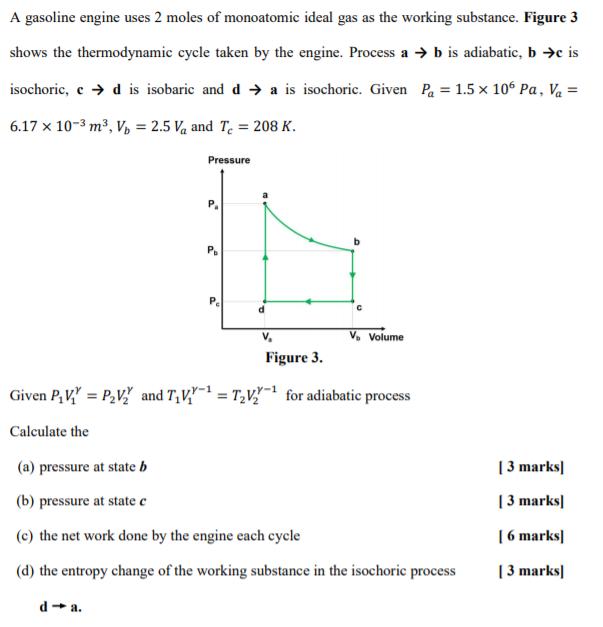
A gasoline engine uses 2 moles of monoatomic ideal gas as the working substance. Figure 3 shows the thermodynamic cycle taken by the engine. Process a b is adiabatic, b c is isochoric, e d is isobaric and d a is isochoric. Given Pa = 1.5 x 10 Pa, Va = 6.17 x 10-3 m, V, = 2.5 Va and T = 208 K. Pressure V, V. Volume Figure 3. Given P, V" = P,V" and T,V = T,V for adiabatic process Calculate the (a) pressure at state b [ 3 marks] (b) pressure at state c | 3 marks| (c) the net work done by the engine each cycle | 6 marks| (d) the entropy change of the working substance in the isochoric process [ 3 marks] d - a.
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
6363e9b05f877_238557.pdf
180 KBs PDF File
6363e9b05f877_238557.docx
120 KBs Word File


