Question: A general reaction written as: 2A + 2B + C +2D, is studied and yield the following data ;- O EXP.1: [A] 0,1M; [B] o
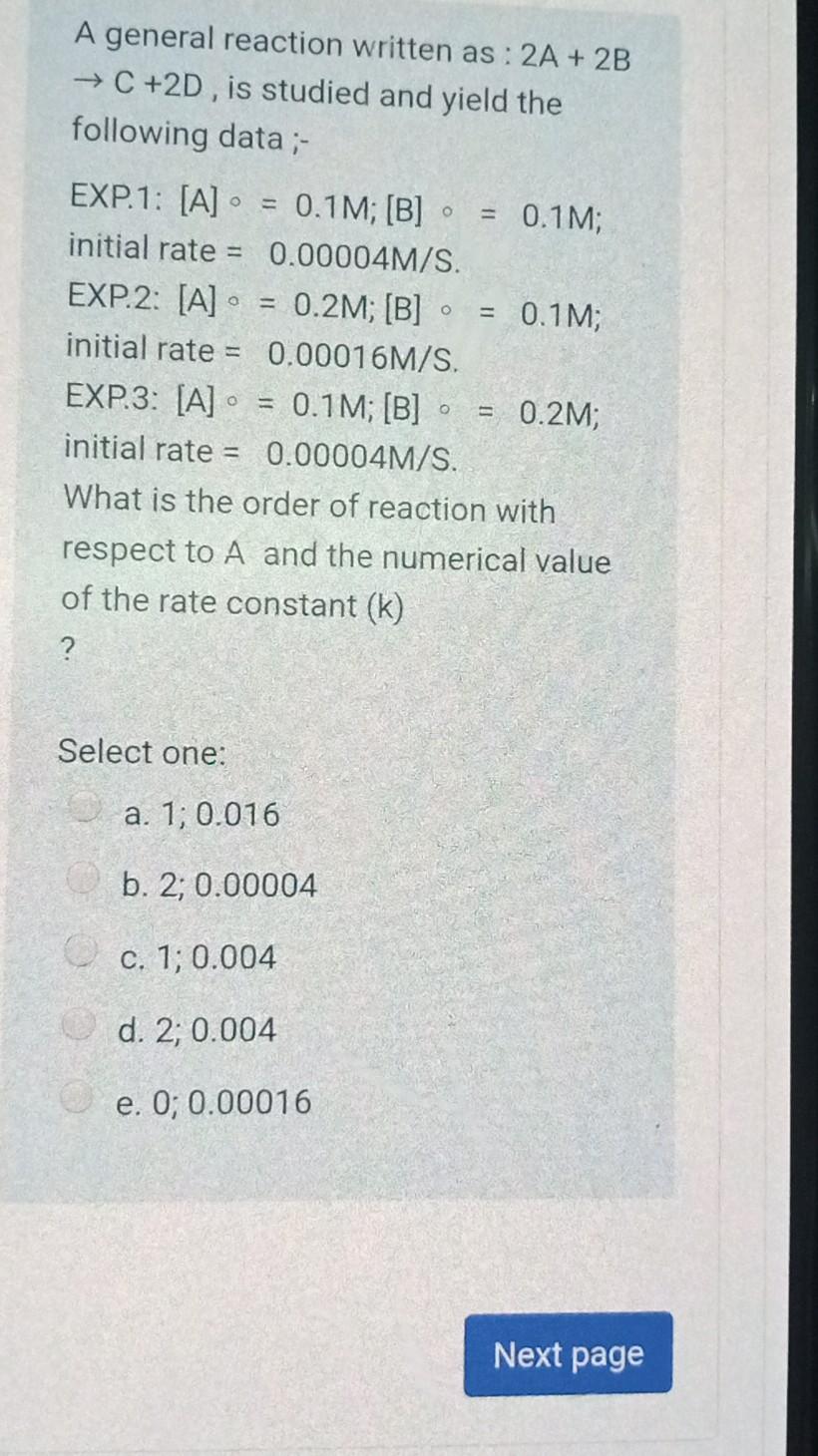
A general reaction written as: 2A + 2B + C +2D, is studied and yield the following data ;- O EXP.1: [A] 0,1M; [B] o = 0,1M; initial rate = 0.00004M/S. EXP.2: [A] = 0.2M; [B] = 0.1M; initial rate = 0.00016M/S. EXP.3: [A] = 0.1M; [B] = 0.2M; initial rate = 0.00004M/S. What is the order of reaction with respect to A and the numerical value of the rate constant (k) ? Select one: a. 1; 0.016 b. 2; 0.00004 c. 1; 0.004 d. 2; 0.004 e. O; 0.00016 Next page
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


