Question: A generic reactoin has the balanced equation: 3A+4BC+5D If you have 0.25mol of A, how many mol of D are produced (assume excess B )?
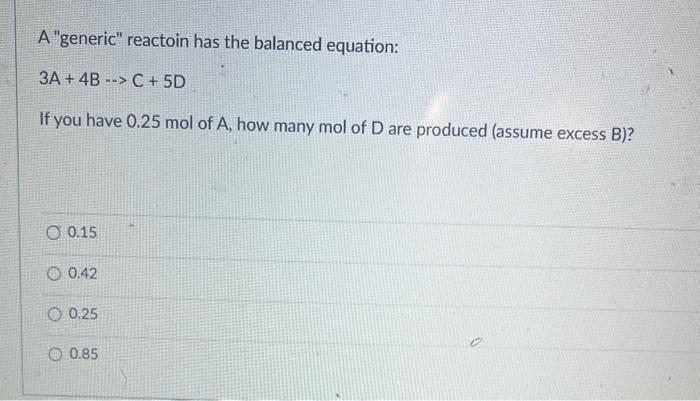
A "generic" reactoin has the balanced equation: 3A+4BC+5D If you have 0.25mol of A, how many mol of D are produced (assume excess B )? 0.15 0.42 0.25 0.85
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


