Question: A hypothetical metal has a cubic unit cell, a density of 7.30 g/cm, a coordination number of 6, and an atomic weight of 94.76
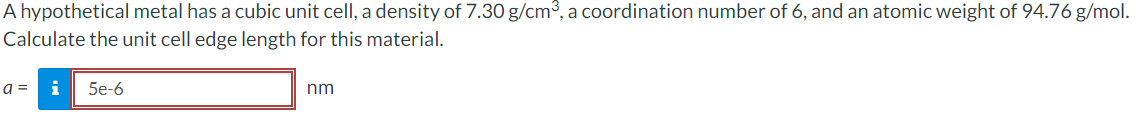
A hypothetical metal has a cubic unit cell, a density of 7.30 g/cm, a coordination number of 6, and an atomic weight of 94.76 g/mol. Calculate the unit cell edge length for this material. a = i 5e-6 nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


