Question: (a) Identify and write oxidation and reduction half-cell reactions for the following equation. (2 marks) Cu(s)+2Agaq+Cuaq2++2Ag(s) (b) Balance the following redox equation (3 marks) Ceaq4++Snaq2+Ceaq3++Snaq4+
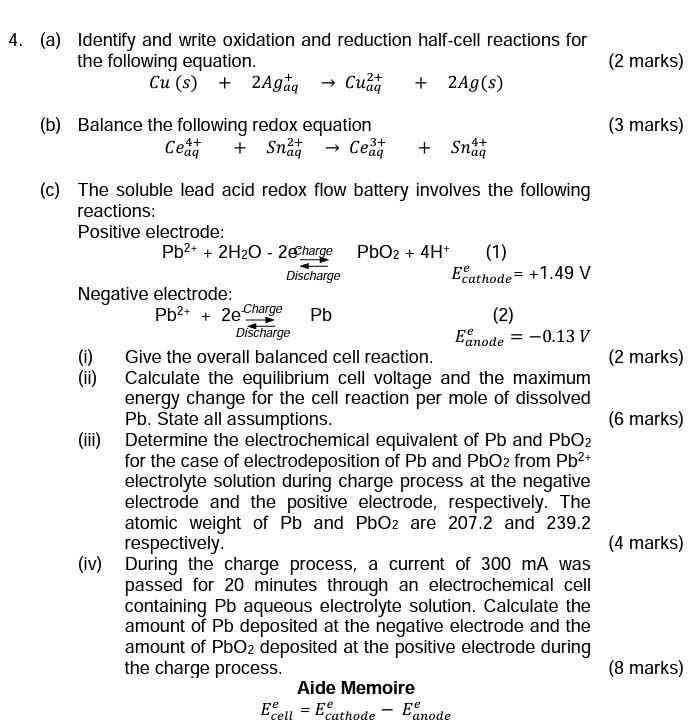
(a) Identify and write oxidation and reduction half-cell reactions for the following equation. (2 marks) Cu(s)+2Agaq+Cuaq2++2Ag(s) (b) Balance the following redox equation (3 marks) Ceaq4++Snaq2+Ceaq3++Snaq4+ (c) The soluble lead acid redox flow battery involves the following reactions: Positive electrode: Negative electrode: Pb2++2DischargeChargePbEanodee=0.13V (i) Give the overall balanced cell reaction. (2 marks) energy change for the cell reaction per mole of dissolved Pb. State all assumptions. (6 marks) (iii) Determine the electrochemical equivalent of Pb and PbO2 for the case of electrodeposition of Pb and PbO2 from Pb2+ electrolyte solution during charge process at the negative electrode and the positive electrode, respectively. The atomic weight of Pb and PbO2 are 207.2 and 239.2 respectively. (4 marks) (iv) During the charge process, a current of 300mA was passed for 20 minutes through an electrochemical cell containing Pb aqueous electrolyte solution. Calculate the amount of Pb deposited at the negative electrode and the amount of PbO2 deposited at the positive electrode during the charge process. (8 marks) Aide Memoire Ecelle=EcathodeeEanodee
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


