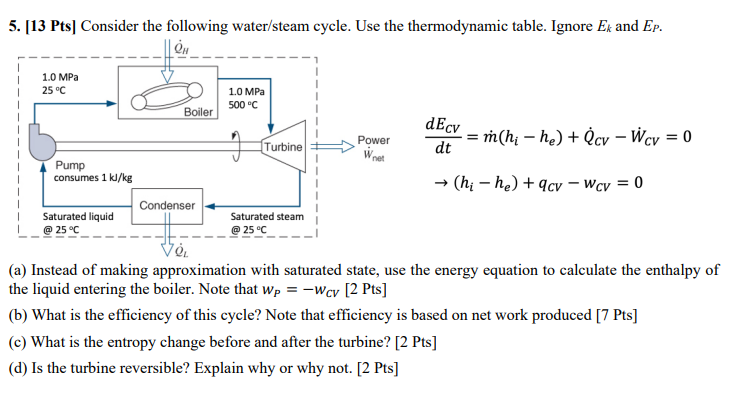
Question: ( a ) Instead of making approximation with saturated state, use the energy equation to calculate the enthalpy of the liquid entering the boiler. Note
a Instead of making approximation with saturated state, use the energy equation to calculate the enthalpy of the liquid entering the boiler. Note that wPwC V Pts
b What is the efficiency of this cycle? Note that efficiency is based on net work produced Pts
c What is the entropy change before and after the turbine? Pts
d Is the turbine reversible? Explain why or why not. Pts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


