Question: = = A laboratory provides the following analysis obtained from a 50 mL sample of raw water. [Ca2+] = 56 mg/L, [Mg2+] = 11 mg/L,
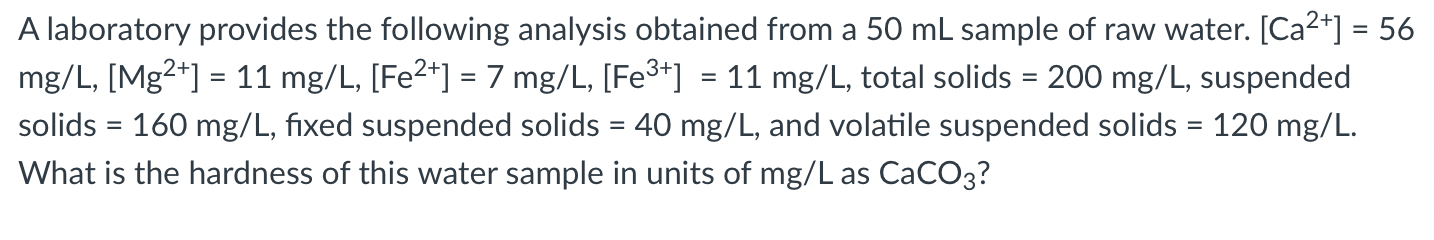
= = A laboratory provides the following analysis obtained from a 50 mL sample of raw water. [Ca2+] = 56 mg/L, [Mg2+] = 11 mg/L, [Fe2+] = 7 mg/L, [Fe3+] = 11 mg/L, total solids = 200 mg/L, suspended solids = 160 mg/L, fixed suspended solids = 40 mg/L, and volatile suspended solids = 120 mg/L. What is the hardness of this water sample in units of mg/L as CaCO3? =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


