Question: A liquid reaction A+B->C takes place in three CSTR's operating in series. The rate expression for this reaction is given to be as: -r_(A)=(k_(1)C_(A)C_(B))/(1+k_(2)C_(c)),(mo(l)/( l)t.min)
A liquid reaction
A+B->Ctakes place in three CSTR's operating in series. The rate\ expression for this reaction is given to be as:\
-r_(A)=(k_(1)C_(A)C_(B))/(1+k_(2)C_(c)),(mo(l)/( l)t.min) \ The concentration of
A,Band
Cat the inlet of the reactor are
C_(A0)=C_(B0)=1Mand\
C_(C0)=0. The volumetric flow rate of the inlet stream is
50l(t)/(m)in. All reactors operate at\
50\\\\deg C. The volume of the fist reactor is
50ltand the volume of the second and the third\ reactors are
75Iteach. Find the concentration of
Cat the outlet of the second and third\ reactors.\
k_(1)=10^(16)e^(-(12000)/(T))\ k_(2)=10^(7)e^(-(5000)/(T)) 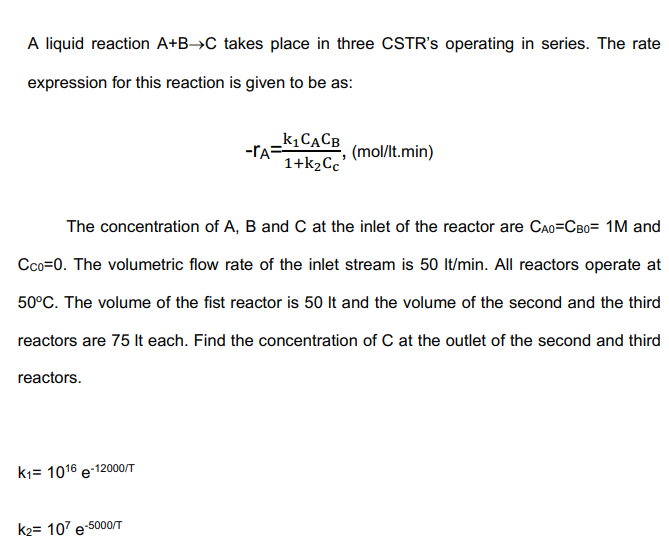
A liquid reaction A+BC takes place in three CSTR's operating in series. The rate expression for this reaction is given to be as: rA=1+k2Cck1CACB,(mol/lt.min) The concentration of A,B and C at the inlet of the reactor are CA0=CB0=1M and Cc0=0. The volumetric flow rate of the inlet stream is 50lt/min. All reactors operate at 50C. The volume of the fist reactor is 50lt and the volume of the second and the third reactors are 75It each. Find the concentration of C at the outlet of the second and third reactors. k1=1016e12000/T k2=107e5000/T A liquid reaction A+BC takes place in three CSTR's operating in series. The rate expression for this reaction is given to be as: rA=1+k2Cck1CACB,(mol/lt.min) The concentration of A,B and C at the inlet of the reactor are CA0=CB0=1M and Cc0=0. The volumetric flow rate of the inlet stream is 50lt/min. All reactors operate at 50C. The volume of the fist reactor is 50lt and the volume of the second and the third reactors are 75It each. Find the concentration of C at the outlet of the second and third reactors. k1=1016e12000/T k2=107e5000/T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


