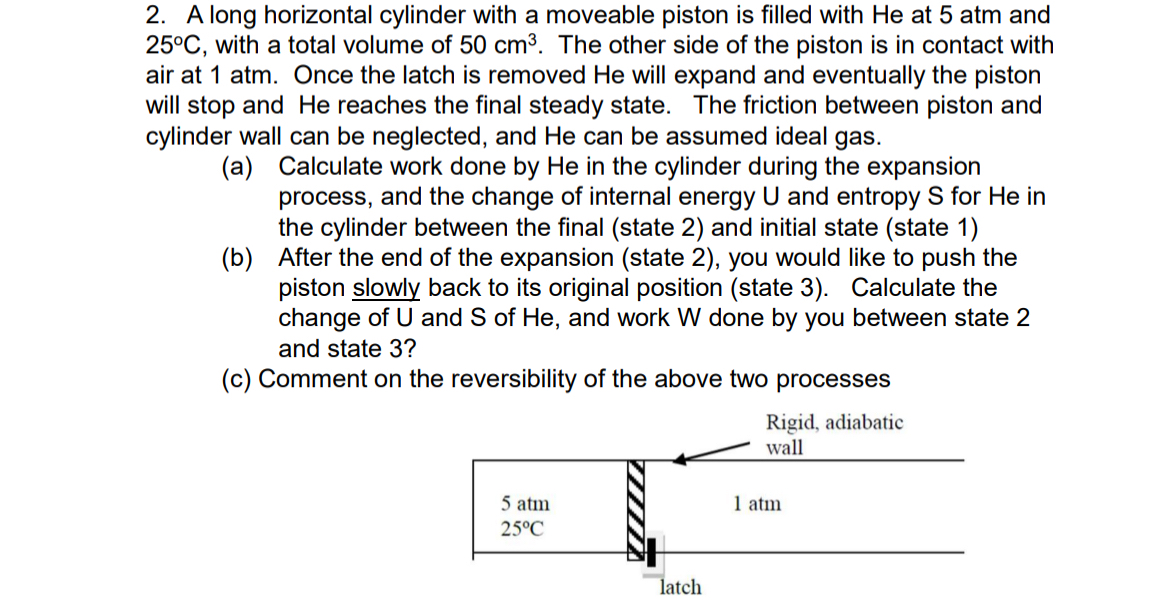
Question: A long horizontal cylinder with a moveable piston is filled with He at 5 atm and 2 5 C , with a total volume of
A long horizontal cylinder with a moveable piston is filled with He at atm and
with a total volume of The other side of the piston is in contact with
air at atm Once the latch is removed He will expand and eventually the piston
will stop and He reaches the final steady state. The friction between piston and
cylinder wall can be neglected, and He can be assumed ideal gas.
a Calculate work done by He in the cylinder during the expansion
process, and the change of internal energy and entropy for He in
the cylinder between the final state and initial state state
b After the end of the expansion state you would like to push the
piston slowly back to its original position state Calculate the
change of and of He and work done by you between state
and state
c Comment on the reversibility of the above two processes
please solve on a paper so its easy to comprehend
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


