Question: a) Make appropriate plots or perform linear regression using these data to determine the reaction order. b) Determine the rate constant for the reaction. c)
a) Make appropriate plots or perform linear regression using these data to determine the reaction order.
b) Determine the rate constant for the reaction. c) Using the rate law you have determined, calculate the half-life for the reaction. d) Calculate how long it will take for the concentration of X to be 0.33 M.
the data is as follows :
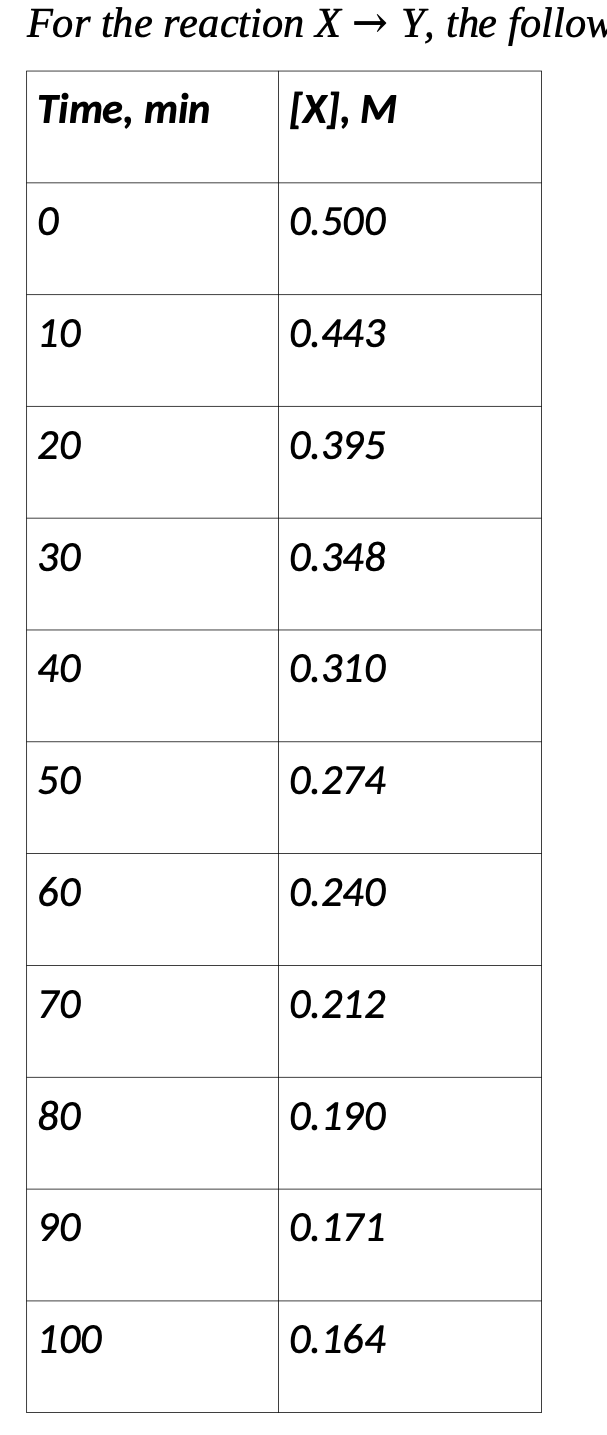
For the reaction XY, the follow Time, min [X], M 0 0.500 10 0.443 20 0.395 30 0.348 40 0.310 50 0.274 60 0.240 70 0.212 80 0.190 90 0.171 100 0.164
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


