Question: A metallic solid ingot has a mass of 1 4 . 7 grams. It was loaded into a graduated cylinder containing 4 0 . 0
A metallic solid ingot has a mass of grams. It was loaded into a graduated cylinder containing or water. Upon addition of the ingot, the volume of the water and the ingot was What is the density of the ingot?
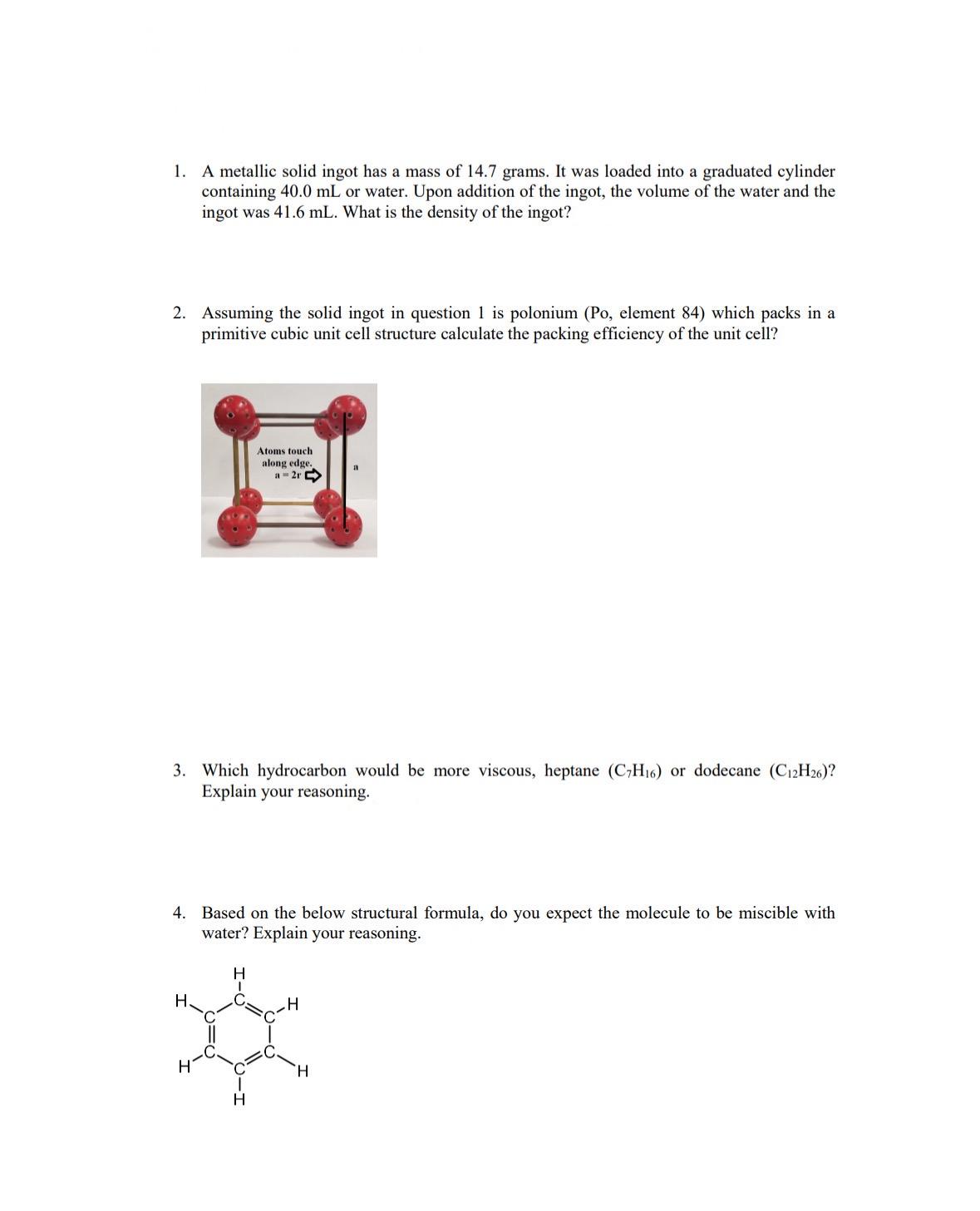
Assuming the solid ingot in question is polonium Po element which packs in a primitive cubic unit cell structure calculate the packing efficiency of the unit cell?
Which hydrocarbon would be more viscous, heptane or dodecane Explain your reasoning.
Based on the below structural formula, do you expect the molecule to be miscible with water? Explain your reasoning.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


