Question: A mixture of methanol and sodium phosphate buffer solution (pH 2.6) is used as the mobile phase to separate phenol, benzoic acid and p-toluic acid
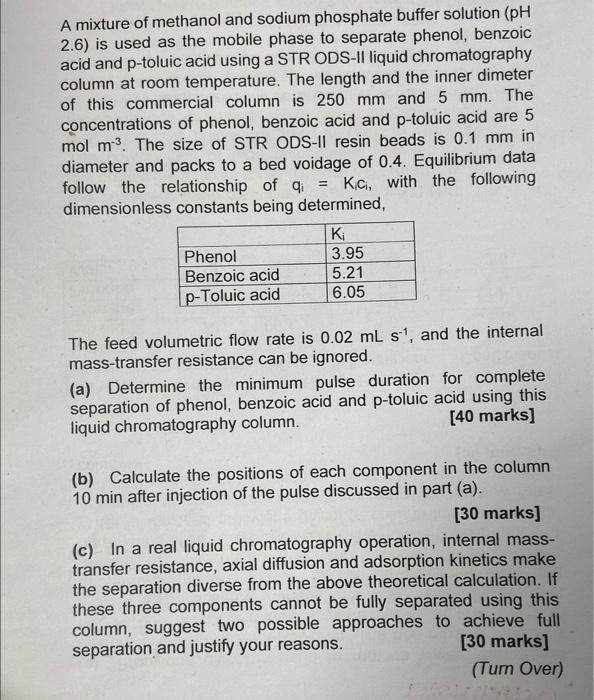
A mixture of methanol and sodium phosphate buffer solution (pH 2.6) is used as the mobile phase to separate phenol, benzoic acid and p-toluic acid using a STR ODS-II liquid chromatography column at room temperature. The length and the inner dimeter of this commercial column is 250mm and 5mm. The concentrations of phenol, benzoic acid and p-toluic acid are 5 mol m3. The size of STR ODS-II resin beads is 0.1mm in diameter and packs to a bed voidage of 0.4. Equilibrium data follow the relationship of qi=Kici, with the following dimensionless constants being determined, The feed volumetric flow rate is 0.02mLs1, and the internal mass-transfer resistance can be ignored. (a) Determine the minimum pulse duration for complete separation of phenol, benzoic acid and p-toluic acid using this liquid chromatography column. [40 marks] (b) Calculate the positions of each component in the column 10min after injection of the pulse discussed in part (a). [30 marks] (c) In a real liquid chromatography operation, internal masstransfer resistance, axial diffusion and adsorption kinetics make the separation diverse from the above theoretical calculation. If these three components cannot be fully separated using this column, suggest two possible approaches to achieve full separation and justify your reasons. [30 marks] (Turn Over)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


