Question: A molecule has with the formula CnH2n+2 will have the same DOU as a compound with which of the following formulas? C2H, O CH3n-2 O
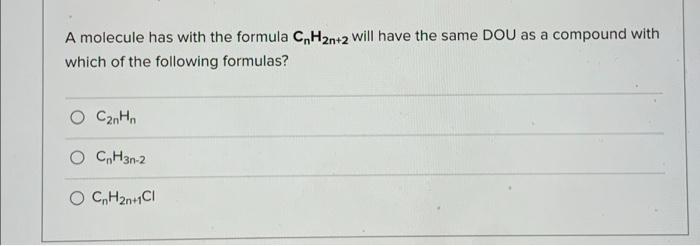

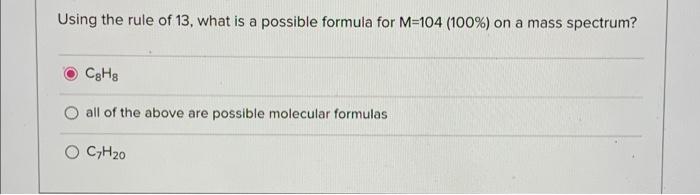

A molecule has with the formula CnH2n+2 will have the same DOU as a compound with which of the following formulas? C2H, O CH3n-2 O C, H2n+1C1 Mass spec: M = 152 (100%), 154 (66%), 156 (11%). Which of the following is a possible molecular formula? O CsHgNCI2 C6H10Cl2 O all of the above are possible molecular formulas Using the rule of 13, what is a possible formula for M=104 (100%) on a mass spectrum? CgHg all of the above are possible molecular formulas CHO Mass spec: M = 379 (100%), 381 (98%). Which of the following is a possible molecular formula? all of the above are possible molecular formulas C22H22NBI C21H19NOBI
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


