Question: A monatomic ideal gas expands from point A to point B along the path shown in the drawing ( a ) Determine the total work
A monatomic ideal gas expands from point A to point B along the path shown in the drawinga Determine the total work done by the gas b The temperature of the gas point A is K What is its temperature at point Bc How much heat has been added to or removed from the gas during the process? Hint : Use ideal gas equation pVnRT points A and B
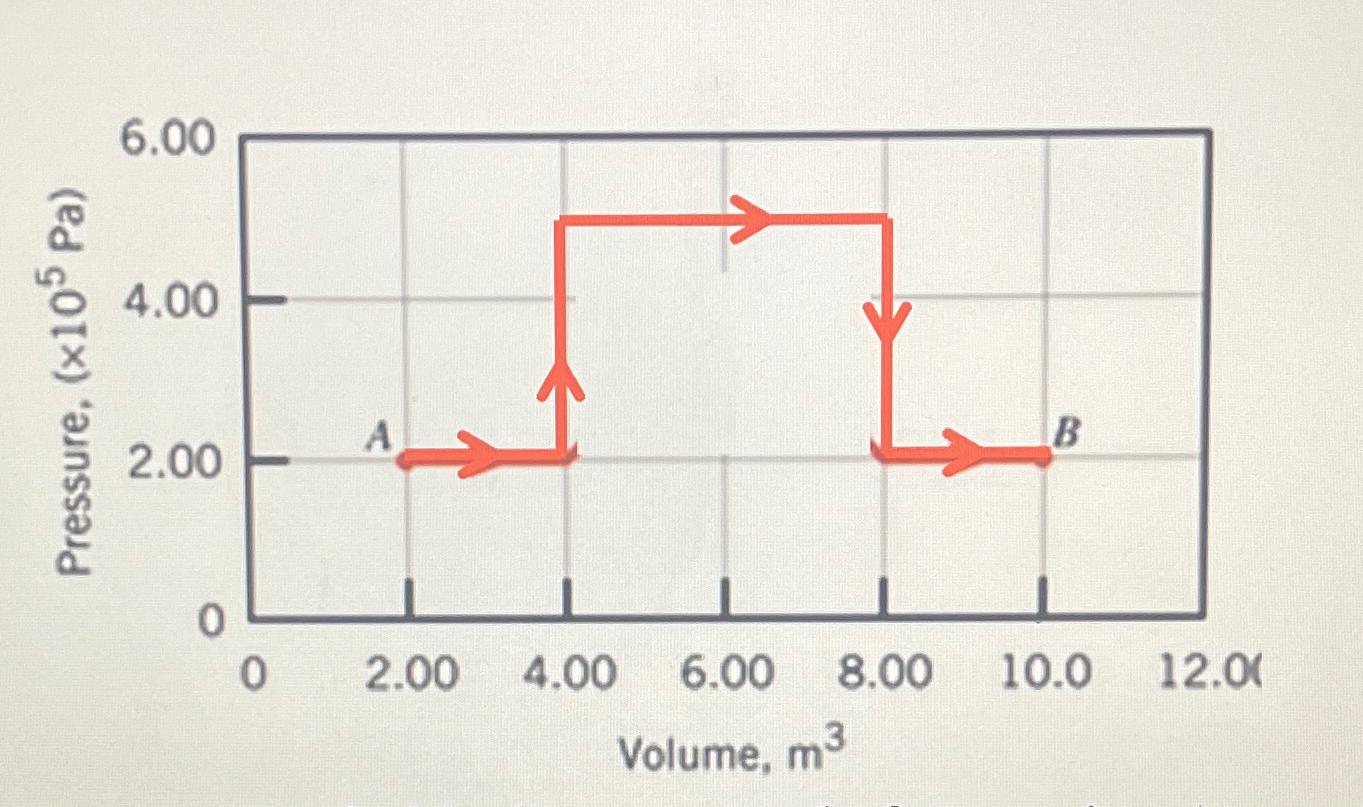
Pressure, (105 Pa) 6.00 4.00 A 2.00 0 0 2.00 4.00 6.00 Volume, m B 8.00 10.0 12.00
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


