Question: a) NEED TO SOLVE THIS PROBLEM BY RK4 METHOD B) DERIVE SYSTEM OF ODEs c) DESCRIBE HOW TO USE EACH SET OF ODEs to solve
 a) NEED TO SOLVE THIS PROBLEM BY RK4 METHOD
a) NEED TO SOLVE THIS PROBLEM BY RK4 METHOD
B) DERIVE SYSTEM OF ODEs
c) DESCRIBE HOW TO USE EACH SET OF ODEs to solve the problem.
d) CLEARLY EXPLAIN HOW TO APPLY RK4 to get the desired result.
e) SHOW AN EXAMPLE OF EACH ITERATION FROM WHERE I CAN CONTINUE TO SOLVE THE PROBLEM.
F) ANSWER SHOULD CLEARLY HELP IN GETTING THE FINAL RESULT
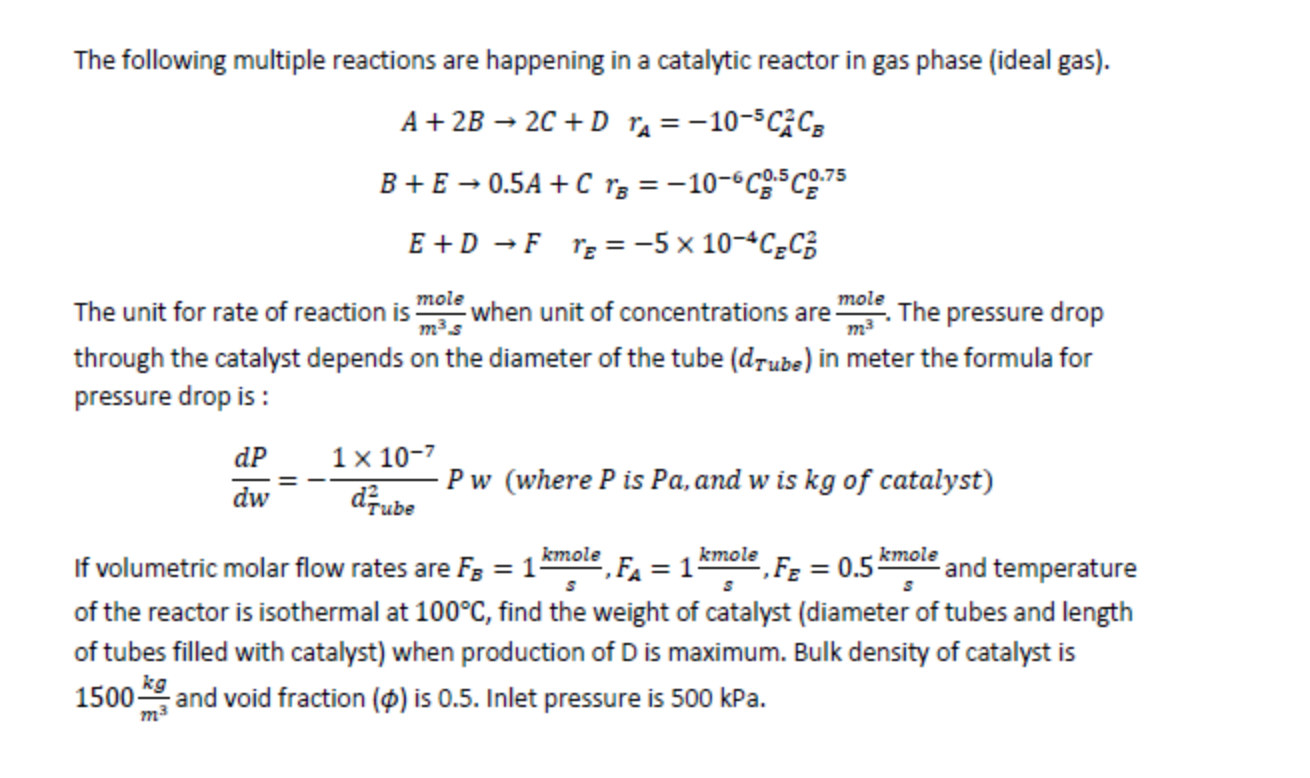
The following multiple reactions are happening in a catalytic reactor in gas phase (ideal gas). A+2B2C+DrA=105CA2CBB+E0.5A+CrB=106CB0.5CE0.75E+DFrE=5104CECD2 The unit for rate of reaction is m3smole when unit of concentrations are m3mole. The pressure drop through the catalyst depends on the diameter of the tube (dTube) in meter the formula for pressure drop is : dwdP=dTube21107Pw(wherePisPa,andwiskgofcatalyst) If volumetric molar flow rates are FB=1skmole,FA=1skmole,FE=0.5skmole and temperature of the reactor is isothermal at 100C, find the weight of catalyst (diameter of tubes and length of tubes filled with catalyst) when production of D is maximum. Bulk density of catalyst is 1500m3kg and void fraction () is 0.5 . Inlet pressure is 500kPa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


